BROMFED DM- brompheniramine maleate, pseudoephedrine hydrochloride and dextromethorphan hydrobromide syrup
Bromfed DM by
Drug Labeling and Warnings
Bromfed DM by is a Prescription medication manufactured, distributed, or labeled by Morton Grove Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Bromfed® DM Cough Syrup is a clear, light pink syrup with a butterscotch flavor.
Each 5 mL (1 teaspoonful) contains:
Brompheniramine Maleate, USP . . . . . . . . 2 mg
Pseudoephedrine Hydrochloride, USP . . . . . 30 mg
Dextromethorphan Hydrobromide, USP . . . 10 mg
Alcohol 0.95% v/v
In a palatable, aromatic vehicle.
Inactive Ingredients: artificial butterscotch flavor, citric acid anhydrous, dehydrated alcohol, FD&C Red No. 40, glycerin, liquid sugar, methylparaben, propylene glycol, purified water and sodium benzoate. It may contain 10% citric acid solution or 10% sodium citrate solution for pH adjustment. The pH range is between 3.0 and 6.0.
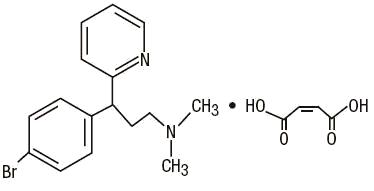
Brompheniramine Maleate, USP(±)-2- p-Bromo-α-2-(dimethylamino)ethylbenzylpyridine maleate (1:1)
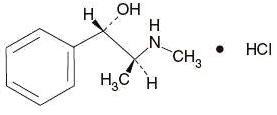
Pseudoephedrine Hydrochloride, USP (+)-Pseudoephedrine hydrochloride
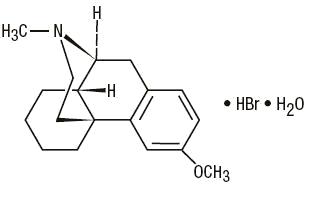
Dextromethorphan Hydrobromide, USP 3-Methoxy-17-methyl-9α, 13α, 14α -morphinan hydrobromide monohydrate
Antihistamine/Nasal Decongestant/Antitussive syrup for oral administration.
-
CLINICAL PHARMACOLOGY
Brompheniramine maleate is a histamine antagonist, specifically an H1-receptor-blocking agent belonging to the alkylamine class of antihistamines. Antihistamines appear to compete with histamine for receptor sites on effector cells. Brompheniramine also has anticholinergic (drying) and sedative effects. Among the antihistaminic effects, it antagonizes the allergic response (vasodilation, increased vascular permeability, increased mucus secretion) of nasal tissue. Brompheniramine is well absorbed from the gastrointestinal tract, with peak plasma concentration after single, oral dose of 4 mg reached in 5 hours; urinary excretion is the major route of elimination, mostly as products of biodegradation; the liver is assumed to be the main site of metabolic transformation.
Pseudoephedrine acts on sympathetic nerve endings and also on smooth muscle, making it useful as a nasal decongestant. The nasal decongestant effect is mediated by the action of pseudoephedrine on α-sympathetic receptors, producing vasoconstriction of the dilated nasal arterioles. Following oral administration, effects are noted within 30 minutes with peak activity occurring at approximately one hour.
Dextromethorphan acts centrally to elevate the threshold for coughing. It has no analgesic or addictive properties. The onset of antitussive action occurs in 15 to 30 minutes after administration and is of long duration.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Hypersensitivity to any of the ingredients. Do not use in the newborn, in premature infants, in nursing mothers, or in patients with severe hypertension or severe coronary artery disease. Do not use dextromethorphan in patients receiving monoamine oxidase (MAOI) inhibitors (see PRECAUTIONS - Drug Interactions).
Antihistamines should not be used to treat lower respiratory tract conditions including asthma.
- WARNINGS
-
PRECAUTIONS
Because of its antihistamine component, Bromfed® DM Cough Syrup should be used with caution in patients with a history of bronchial asthma, narrow angle glaucoma, gastrointestinal obstruction, or urinary bladder neck obstruction. Because of its sympathomimetic component, Bromfed® DM Cough Syrup should be used with caution in patients with diabetes, hypertension, heart disease, or thyroid disease.
Patients should be warned about engaging in activities requiring mental alertness, such as driving a car or operating dangerous machinery.
Monoamine oxidase (MAO) inhibitors
Hyperpyrexia, hypotension, and death have been reported coincident with the coadministration of MAO inhibitors and products containing dextromethorphan. In addition, MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines and may enhance the effect of pseudoephedrine. Concomitant administration of Bromfed® DM Cough Syrup and MAO inhibitors should be avoided (see CONTRAINDICATIONS).
Central nervous system (CNS) depressants
Antihistamines have additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, antianxiety agents, etc.).
Antihypertensive drugs
Sympathomimetic may reduce the effects of antihypertensive drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies of Bromfed® DM Cough Syrup to assess the carcinogenic and mutagenic potential or the effect on fertility have not been performed.
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Bromfed® DM Cough Syrup. It is also not known whether Bromfed® DM Cough Syrup can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. It should be given to a pregnant woman only if clearly needed.
Reproduction studies of brompheniramine maleate (a component of Bromfed® DM Cough Syrup) in rats and mice at doses up to 16 times the maximum human doses have revealed no evidence of impaired fertility or harm to the fetus.
Because of the higher risk of intolerance of antihistamines in small infants generally, and in newborns and prematures in particular, Bromfed® DM Cough Syrup is contraindicated in nursing mothers.
Safety and effectiveness in pediatric patients below the age of 6 months have not been established (see DOSAGE AND ADMINISTRATION).
Clinical studies of Bromfed® DM Cough Syrup did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients. The elderly are also more likely to experience adverse reactions to sympathomimetics.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
The most frequent adverse reactions to Bromfed® DM Cough Syrup are: sedation; dryness of mouth, nose and throat; thickening of bronchial secretions; dizziness. Other adverse reactions may include:
Dermatologic: Urticaria, drug rash, photosensitivity, pruritus.
Cardiovascular System: Hypotension, hypertension, cardiac arrhythmias, palpitation.
CNS: Disturbed coordination, tremor, irritability, insomnia, visual disturbances, weakness, nervousness, convulsions, headache, euphoria, and dysphoria.
G.U. System: Urinary frequency, difficult urination.
G.I. System: Epigastric discomfort, anorexia, nausea, vomiting, diarrhea, constipation.
Respiratory System: Tightness of chest and wheezing, shortness of breath.
Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
-
OVERDOSAGE
Central nervous system effects from overdosage of brompheniramine may vary from depression to stimulation, especially in children. Anticholinergic effects may be noted. Toxic doses of pseudoephedrine may result in CNS stimulation, tachycardia, hypertension, and cardiac arrhythmias; signs of CNS depression may occasionally be seen. Dextromethorphan in toxic doses will cause drowsiness, ataxia, nystagmus, opisthotonos, and convulsive seizures.
Data suggest that individuals may respond in an unexpected manner to apparently small amounts of a particular drug. A 2½-year-old child survived the ingestion of 21 mg/kg of dextromethorphan exhibiting only ataxia, drowsiness, and fever, but seizures have been reported in 2 children following the ingestion of 13–17 mg/kg. Another 2½-year-old child survived a dose of 300–900 mg of brompheniramine. The toxic dose of pseudoephedrine should be less than that of ephedrine, which is estimated to be 50 mg/kg.
Induce emesis if patient is alert and is seen prior to 6 hours following ingestion. Precautions against aspiration must be taken, especially in infants and small children. Gastric lavage may be carried out, although in some instances tracheostomy may be necessary prior to lavage. Naloxone hydrochloride 0.005 mg/kg intravenously may be of value in reversing the CNS depression that may occur from an overdose of dextromethorphan. CNS stimulants may counter CNS depression. Should CNS hyperactivity or convulsive seizures occur, intravenous short-acting barbiturates may be indicated. Hypertensive responses and/or tachycardia should be treated appropriately. Oxygen, intravenous fluids, and other supportive measures should be employed as indicated.
-
DOSAGE AND ADMINISTRATION
Adults and pediatric patients 12 years of age and over: 10 mL (2 teaspoonfuls) every 4 hours. Children 6 to under 12 years of age: 5 mL (1 teaspoonful) every 4 hours. Children 2 to under 6 years of age: 2.5 mL (½ teaspoonful) every 4 hours. Infants 6 months to under 2 years of age: Dosage to be established by a physician.
Do not exceed 6 doses during a 24-hour period.
-
HOW SUPPLIED
Bromfed® DM Cough Syrup is a clear, light pink-colored, butterscotch-flavored syrup containing in each 5 mL (1 teaspoonful) brompheniramine maleate 2 mg, pseudoephedrine hydrochloride 30 mg and dextromethorphan hydrobromide 10 mg, available in the following sizes:
4 fl oz (118 mL) NDC: 60432-837-04
1 Pint (473 mL) NDC: 60432-837-16
-
RECOMMENDED STORAGE
Store at 20 ° to 25 °C (68 ° to 77 °F) [See USP Controlled Room Temperature].
KEEP TIGHTLY CLOSED
Dispense in a tight, light-resistant container as defined in the USP.
Rx Only
Product No.: 8837
BROMFED® is a registered trademark of Wockhardt.

Wockhardt USA, LLC
Parsippany, NJ 07054
Manufactured By:
Morton Grove Pharmaceuticals, Inc.
Morton Grove, IL 60053
A50-8837-16
REV. 08-15
-
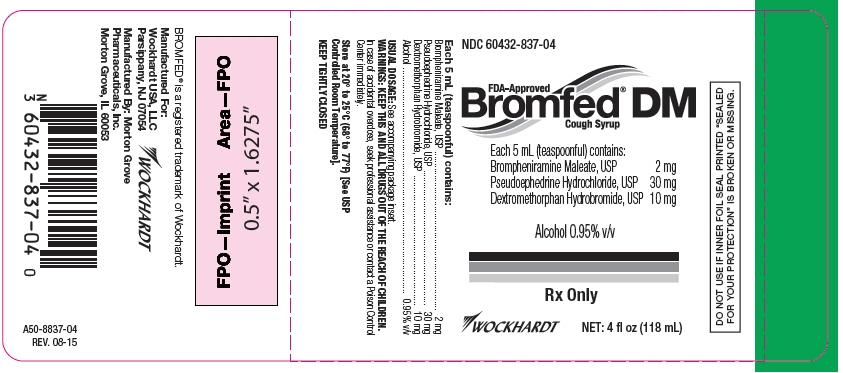
PRINCIPAL DISPLAY PANEL Bottle Label
NDC: 60432-837-04
FDA-Approved
Bromfed® DM
Cough Syrup
Each 5 mL (teaspoonful) contains:
Brompheniramine Maleate, USP 2 mg
Pseudoephedrine Hydrochloride, USP 30 mg
Dextromethorphan Hydrobromide, USP 10 mg
Alcohol 0.95% v/v
Rx Only
WOCKHARDT
NET: 4 fl oz (118 mL)
DO NOT USE IF INNER FOIL SEAL PRINTED "SEALED
FOR YOUR PROTECTION" IS BROKEN OR MISSING.
-
INGREDIENTS AND APPEARANCE
BROMFED DM
brompheniramine maleate, pseudoephedrine hydrochloride and dextromethorphan hydrobromide syrupProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60432-837 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BROMPHENIRAMINE MALEATE (UNII: IXA7C9ZN03) (BROMPHENIRAMINE - UNII:H57G17P2FN) BROMPHENIRAMINE MALEATE 2 mg in 5 mL PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 30 mg in 5 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) FD&C RED NO. 40 (UNII: WZB9127XOA) SUCROSE (UNII: C151H8M554) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Product Characteristics Color PINK (Light Pink) Score Shape Size Flavor BUTTERSCOTCH Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60432-837-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/15/2010 2 NDC: 60432-837-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/15/2010 3 NDC: 60432-837-15 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/15/2010 07/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA088811 06/15/2010 Labeler - Morton Grove Pharmaceuticals, Inc. (801897505) Registrant - Morton Grove Pharmaceuticals, Inc. (801897505)
Trademark Results [Bromfed DM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BROMFED DM 74047192 1638857 Live/Registered |
Muro Pharmaceutical, Inc. 1990-04-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.