the Honey Pot Anti-Itch
Anti-Itch Wipes by
Drug Labeling and Warnings
Anti-Itch Wipes by is a Otc medication manufactured, distributed, or labeled by Diamond Wipes International. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTI-ITCH WIPES- anti-itch wipe cloth
Diamond Wipes International
----------
the Honey Pot
Anti-Itch
Stop use and ask doctor if:
Condition worsens
If symptoms persist for more than 7 days, or clear up and reoccur again within a few days
Keep out of reach of children.
Keep out of reach of of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions:
Adults and children 12 years of age and older: Apply to affected area not more than 3 or 4 times daily.
Children under 12 years of age: Consult a doctor
Inactive ingredients:
Inactive Ingredients: Aloe Barbadensis Leaf Extract, *Aloe Barbadensis Leaf Juice, Althaea officinalis Root Extract, Avena Sativa (Oat) Kernel Extract, Calendula Officinalis Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Cocamidopropyl PG-Dimonium Chloride Phosphate, Cocos Nucifera (Coconut) Fruit Extract, Cucumis Sativus (Cucumber) Fruit
Extract, Disodium Cocoamphodiacetate, Ethylhexylglycerin, Euterpe Oleracea Fruit Extract, Fragrance, Glycerin, Hamamelis Virginiana (Witch Hazel) Water, Helianthus Annuus (Sunflower) Seed Oil, *Honey, Lactobacillus, Lactobacillus Ferment, Lavandula Angustifolia (Lavender) Oil, Phenoxyethanol, Polyglyceryl-4 Caprate, Punica Granatum Fruit Extract, Rosa Centifolia Flower Water, Sodium Chloride, Tetrasodium Glutamate Diacetate Tocopheryl Acetate, *Apple Cider Vinegar, Water
*Organic Ingredients
Label
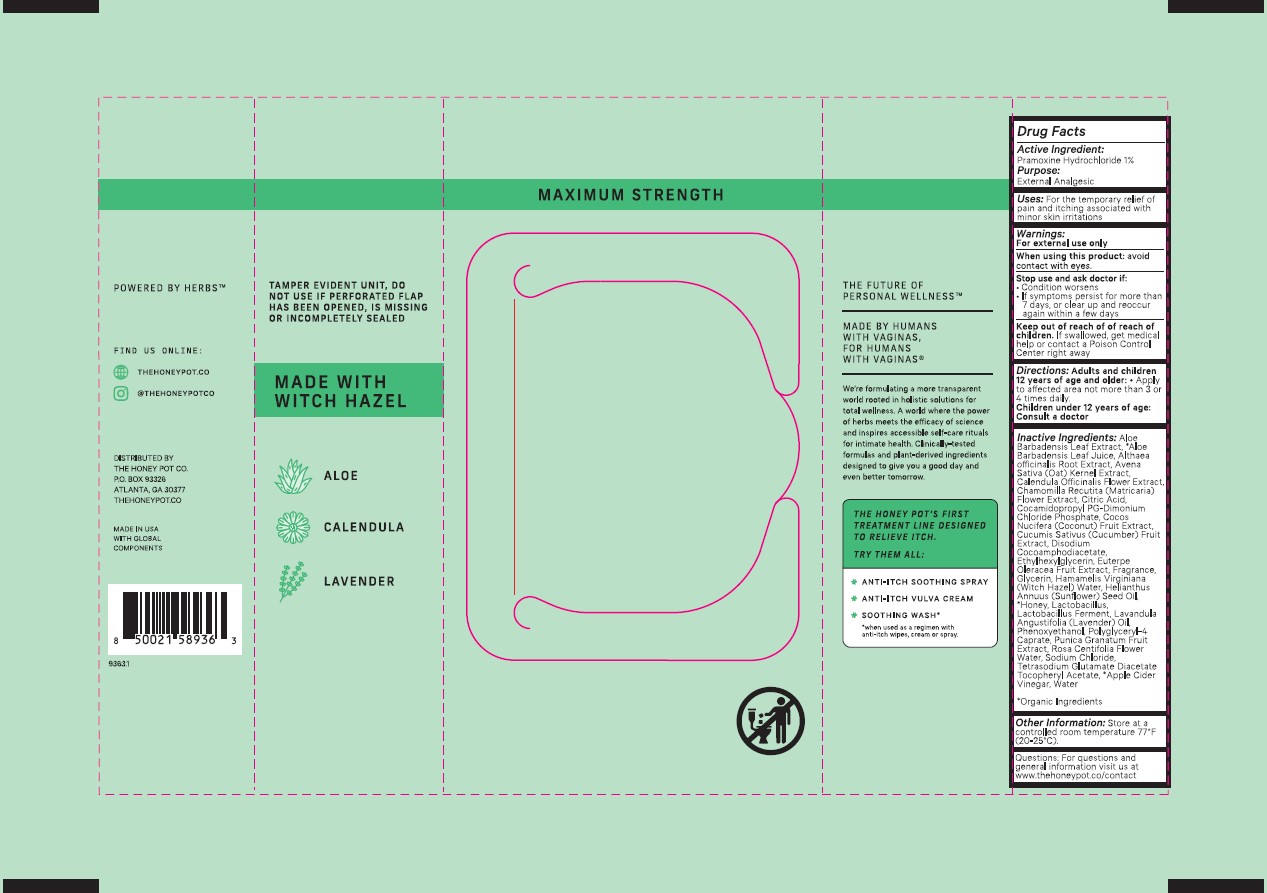
MAXIMUM STRENGTH
the Honey Pot ® company
PLANT-DERIVED FEMININE CARE™
RELIEF
ANTI ITCH
soothing wipes
1% PRAMOXINE HYDROCHLORIDE
(EXTERNAL ANALGESIC)
for temporary relief of itching and discomfort
30 COUNT
9363.1
POWERED BY HERBS™
FIND US ONLINE:
THEHONEYPOT.CO
@THEHONEYPOTCO
DISTRIBUTED BY THE HONEY POT CO.
P.O. BOX 93326 ATLANTA, GA 30377
THEHONEYPOT.CO
MADE IN USA WITH GLOBAL COMPONENTS
8 50021 58936 3
9363.1
TAMPER EVIDENT UNIT, DO NOT USE IF PERFORATED FLAP HAS BEEN OPENED, IS MISSING OR INCOMPLETELY SEALEDS
MADE WITH WITCH HAZEL
ALOE
CALENDULA
LAVENDER
THE FUTURE OF
PERSONAL WELLNESS™
MADE BY HUMANS WITH VAGINAS, FOR HUMANS WITH VAGINAS ®
We’re formulating a more transparent world rooted in holistic solutions for total wellness. A world where the power of herbs meets the efficacy of science and inspires accessible self-care rituals for intimate health. Clinically-tested formulas and plant-derived ingredients designed to give you a good day and even better tomorrow.
THE HONEY POT’S FIRST TREATMENT LINE DESIGNED TO RELIEVE ITCH.
TRY THEM ALL:
♦ ANTI-ITCH SOOTHING SPRAY
♦ ANTI-ITCH VULVA CREAM
♦ SOOTHING WASH*
*when used as a regimen with anti-itch wipes, cream or spray.
Drug Facts
Active Ingredient: Pramoxine Hydrochloride 1%
Purpose: External Analgesic
Uses: For the temporary relief of pain and itching associated with minor skin irritations
Warnings:
For external use only
When using this product: avoid contact with eyes.
Stop use and ask doctor if:
Condition worsens
If symptoms persist for more than 7 days, or clear up and reoccur again within a few days
Keep out of reach of of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions: Adults and children 12 years of age and older: Apply to affected area not more than 3 or 4 times daily.
Children under 12 years of age: Consult a doctor
Inactive Ingredients: Aloe Barbadensis Leaf Extract, *Aloe Barbadensis Leaf Juice, Althaea officinalis Root Extract, Avena Sativa (Oat) Kernel Extract, Calendula Officinalis Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Cocamidopropyl PG-Dimonium Chloride Phosphate, Cocos Nucifera (Coconut) Fruit Extract, Cucumis Sativus (Cucumber) Fruit
Extract, Disodium Cocoamphodiacetate, Ethylhexylglycerin, Euterpe Oleracea Fruit Extract, Fragrance, Glycerin, Hamamelis Virginiana (Witch Hazel) Water, Helianthus Annuus (Sunflower) Seed Oil, *Honey, Lactobacillus, Lactobacillus Ferment, Lavandula Angustifolia (Lavender) Oil, Phenoxyethanol, Polyglyceryl-4 Caprate, Punica Granatum Fruit Extract, Rosa Centifolia Flower Water, Sodium Chloride, Tetrasodium Glutamate Diacetate Tocopheryl Acetate, *Apple Cider Vinegar, Water
*Organic Ingredients
Other Information: Store at a controlled room temperature 77°F (20-25°C).
Questions: For questions and general information visit us at www.thehoneypot.co/contact
the Honey Pot ® company
DESIGNED TO RELIEVE ITCH
CALENDULA ALOE LAVENDER
RELIEF
powered by herbs ®
8 50021 58936 3
RELIEF
| ANTI-ITCH WIPES
anti-itch wipe cloth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Diamond Wipes International (161104729) |