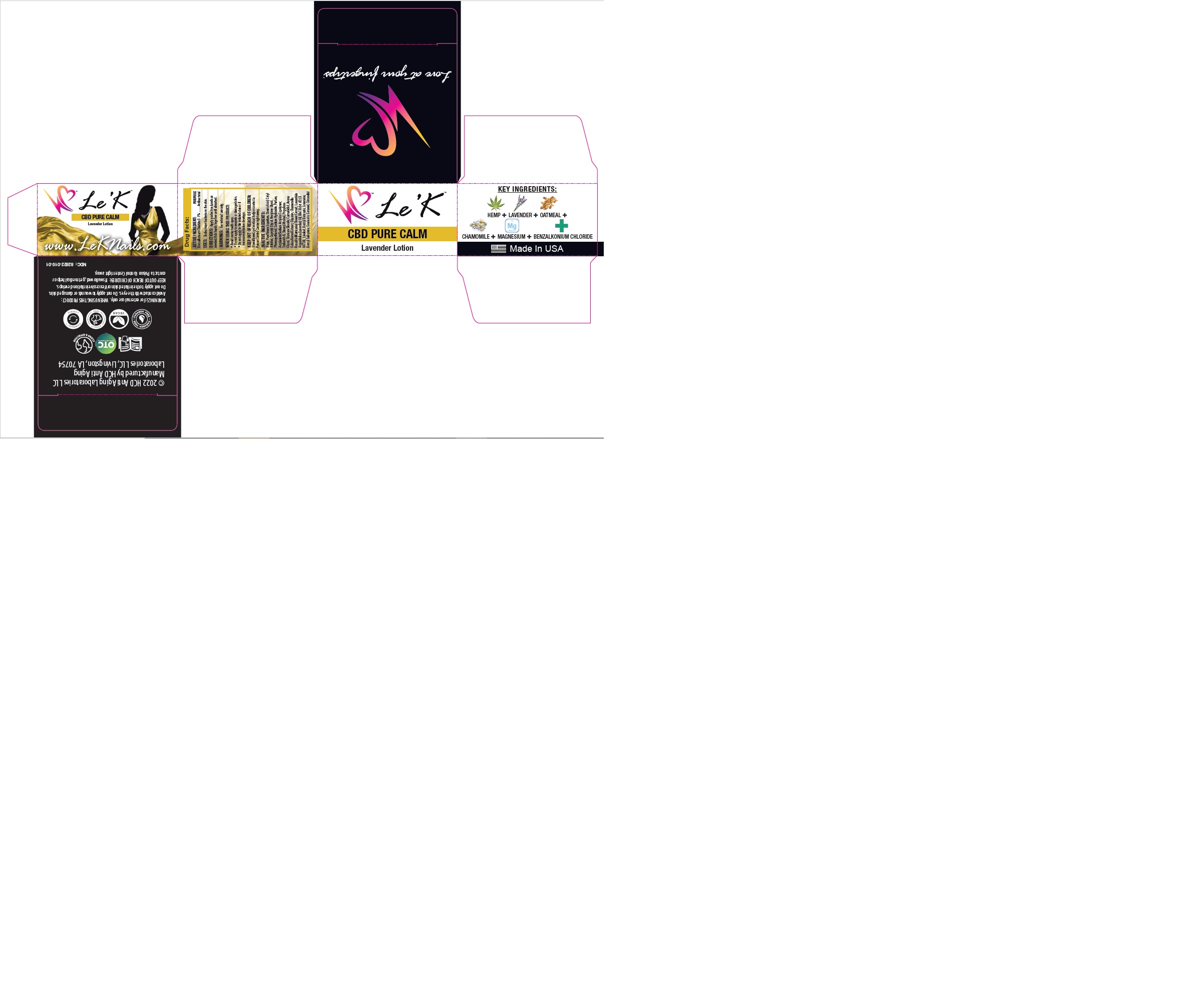
LE K - CBD PURE CALM by HCD ANTI AGING LABORATORIES LLC / CHEMCO CORPORATION 82822-010-01
LE K - CBD PURE CALM by
Drug Labeling and Warnings
LE K - CBD PURE CALM by is a Otc medication manufactured, distributed, or labeled by HCD ANTI AGING LABORATORIES LLC, CHEMCO CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LE K - CBD PURE CALM LAVENDER- benzalkonium chloride lotion
HCD ANTI AGING LABORATORIES LLC
----------
82822-010-01
Avoid contact with the eyes.
Do not apply to wounds or damaged skin.
Do not apply to the irritated skin or if excessive irritation develops.
Aqua, Paraffinum Liquidum, Stearic Acid, Cetyl Alcohol, Caprylyl Glycol, Hexylene Glycol, Phenoxyethanol, Sodium Hydroxide, Parfum, Acrylamide/Sodium Acrylate Copolymer, Trideceth-6, Stearyl Alcohol, Propylene Glycol, Titanium Dioxide, Cannabidiol, Avena Sativa Kernel Extract, Glycerin, Chamomilla Recutita (Matricaria) Flower Extract, Lavandula Angustifolia (Lavender) Oil, D&C Violet #2 (CI 60730), Linalool, Benzyl Benzoate, Limonene, Benzyl Salicylate, Coumarin, Geraniol, Citronellol.
| LE K - CBD PURE CALM
LAVENDER
benzalkonium chloride lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - HCD ANTI AGING LABORATORIES LLC (118675977) |