MOUNTAIN SERIES WEEKENDER MEDICAL- benzalkonium chloride, povidone-iodine, acetaminophen, aspirin, diphenhydramine hydrochloride, ibuprofen, bacitracin zinc, neomycin sulfate, polymyxin b sulfate kit
Mountain Series Weekender Medical by
Drug Labeling and Warnings
Mountain Series Weekender Medical by is a Otc medication manufactured, distributed, or labeled by Tender Corporation dba Adventure Medical Kits. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Use
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- Directions
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
- Warnings
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
- Inactive Ingredient
-
PRINCIPAL DISPLAY PANEL
Genuine Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID.
Triple Antibiotic Ointment 10pcs
Net wt. 0.9g (1/32oz)
100
Triple Antibiotic
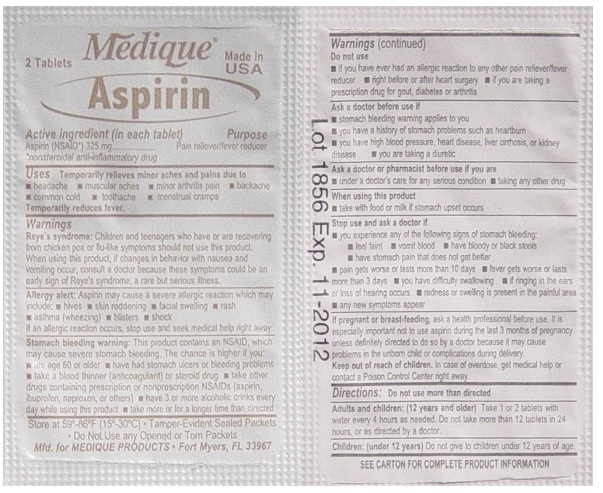
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription
NSAIDs (aspirin, ibuprofen, naproxen, or others) - have 3 or more alcohol drinks every day while
using this product - take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/ fever reducer
- right before or after heart surgery
- if you are taking prescription drugs for gout, diabetes or arthritis
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- if ringing in the ears or loss of hearing occurs redness or swelling is present in the painful area
- any new symptoms appear
-
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless
directed by a doctor (see Warnings) - drink a full glass of water with each dose
- Other information
- Inactive ingredients
- Questions or comments?
- 116R Medique APAP 325 mg Label
- Active ingredients
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking any drugs for asthma
- taking sedatives or tranquilizers
- Directions
- Other information
- Inactive ingredients
- Questions or comments? 1-800-634-7680
-
182R Medique Diphen Label
Collect Medi-Bucks
See inside flap for more details
Medique®
Diphen
Diphenhydramine HCl 25 mg
Hay Fever/Allergies
Fiebre del Heno/Alergias
Pull to Open
TiraParaAbrir
Easy To Swallow Capsules
Capsulas Faciles de Tragar
200 Capsules
(200 x 1)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Untarias
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
- redness or swelling is present in the painful area
- any new or unexpected symptoms occur
- you experience any of the following signs of stomach bleeding:
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- do not use more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directed by a doctor (see Warnings)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive ingredients
carnauba wax*, cellulose*, colloidal silicon dioxide, corn starch*, hypromellose, iron oxide red*, lactose, magnesium stearate, microcrystalline cellulose*, polydextrose, polyethylene glycol, povidone, silica*, sodium lauryl sulfate*, sodium starch glycolate, stearic acid*, titanium dioxide, triacetin*
*may contain
- QUESTIONS
- Principal Display Panel
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Stop using and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- redness or swelling is present
- Directions
- Inactive ingredients
- Questions or comments? 1-800-634-7680
- Principal Display Panel
-
145R Medique APAP 325 mg Label
Collect MediBucks
See inside flap for more details
Medique®
APAP
Acetaminophen 325 mg
Pain Reliever/Fever Reducer
Alivia el Dolor/Reduce la Fiebre
Easy To Swallow
Film Coated Tablets
Facil de Tragar Tabletas con Cubierta Pelicular
Pull to Open
Tire Para Abrir
See new warnings information
500 Tablets
(250 x 2)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Unitarias
- Weekender Kit Label
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS AND PRECAUTIONS
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOUNTAIN SERIES WEEKENDER MEDICAL
benzalkonium chloride, povidone-iodine, acetaminophen, aspirin, diphenhydramine hydrochloride, ibuprofen, bacitracin zinc, neomycin sulfate, polymyxin b sulfate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 44224-0118 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44224-0118-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKAGE 4.8 mL Part 2 3 TUBE 1.5 g Part 3 2 PACKET 4 Part 4 2 PACKET 2 Part 5 1 PACKET 2 Part 6 2 PACKET 4 Part 7 1 PACKET 22 g Part 1 of 7 ANTISEPTIC TOWELETTE
benzalkonium chloride swabProduct Information Item Code (Source) NDC: 52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.40 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/23/2010 Part 2 of 7 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC: 52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52124-0003-1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/16/2010 Part 3 of 7 MEDIQUE ASPIRIN
aspirin tablet, film coatedProduct Information Item Code (Source) NDC: 47682-116 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code 44;157;aspirin Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47682-116-99 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 12/30/2008 Part 4 of 7 MEDIQUE DIPHEN
diphenhydramine hydrochloride capsuleProduct Information Item Code (Source) NDC: 47682-182 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink (pink) , white (white) Score no score Shape CAPSULE (CAPSULE) Size 14mm Flavor Imprint Code CPC;835 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47682-182-46 1 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/30/2008 Part 5 of 7 MEDI-FIRST IBUPROFEN
ibuprofen tablet, film coatedProduct Information Item Code (Source) NDC: 47682-808 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47682-808-99 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075139 12/30/2008 Part 6 of 7 MEDIQUE APAP
acetaminophen tablet, film coatedProduct Information Item Code (Source) NDC: 47682-145 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47682-145-99 2 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 12/30/2008 Part 7 of 7 APLICARE POVIDONE-IODINE SOLUTION
povidone-iodine solution solutionProduct Information Item Code (Source) NDC: 52380-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) POVIDONE-IODINE 9.8 g in 100 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52380-0001-3 22 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333A 08/01/2011 Labeler - Tender Corporation dba Adventure Medical Kits (064437304)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.