Get-Gone - Acne Body Cleanser
Get-Gone - Acne Body Cleanser by
Drug Labeling and Warnings
Get-Gone - Acne Body Cleanser by is a Otc medication manufactured, distributed, or labeled by Maelys Cosmetics, LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
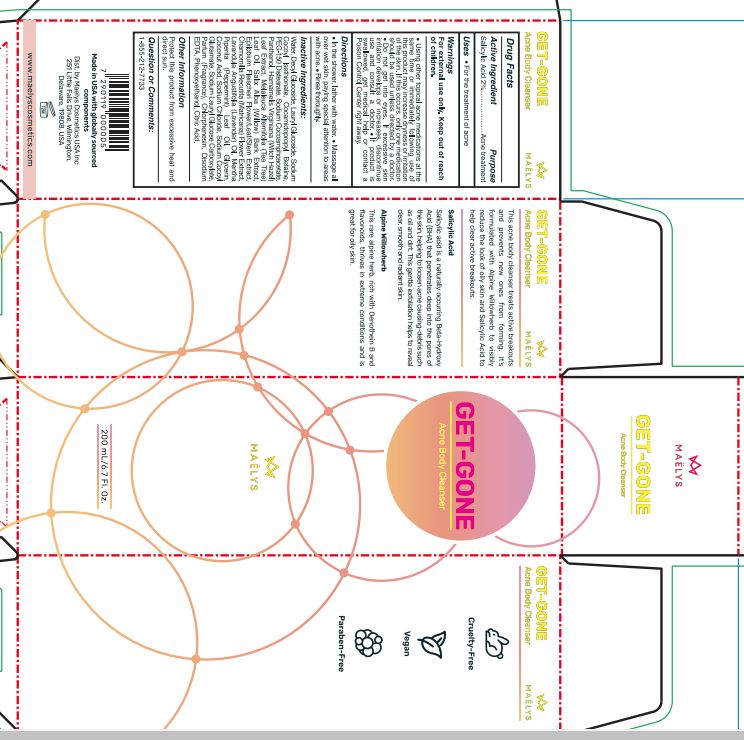
GET-GONE - ACNE BODY CLEANSER- salicylic acid gel
Maelys Cosmetics, LTD
----------
Get-Gone - Acne Body Cleanser
Warnings
For external use only.
Keep out of reach of children.
Using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor. Do not get into eyes. tt excessive skin irritation develops or increases, discontinue use and consult a doctor. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
In the shower, lather with water. Massage all over wet skin, paying special attention to areas with acne. Rinse thoroughly.
Inactive Ingredients:
Water, Decyl Glucoside, Lauryl Glucoside, Sodium Cocoyl isethionate, Cocamidopropyl Betaine, PEG-150 Distearate, Sodium Cocoamphoacetate, Panthenol, Hamamelis Virginiana (Witch Hazel) Leaf Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Salix Alba (Willow) Bark Extract, pilobium Fleischeri Flower/Leaf/Stem Extract, Chamomilla Recutita (Matricaria) Flower Extract, Lavandula Angustifolia (Lavender) Oil, Mentha Piperita (Peppermint) Leaf Oil, Glycerin, Coconut Acid, Sodium Chloride, Sodium Cocoyl Glutamate, Sodium Lauryl Glucose Carboxylate, Parfum (Fragrance), Chlorphenesin, Disodium EDTA, Phenoxyelhanol, Citric Acid.
| GET-GONE - ACNE BODY CLEANSER
salicylic acid gel |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Maelys Cosmetics, LTD (532018258) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.