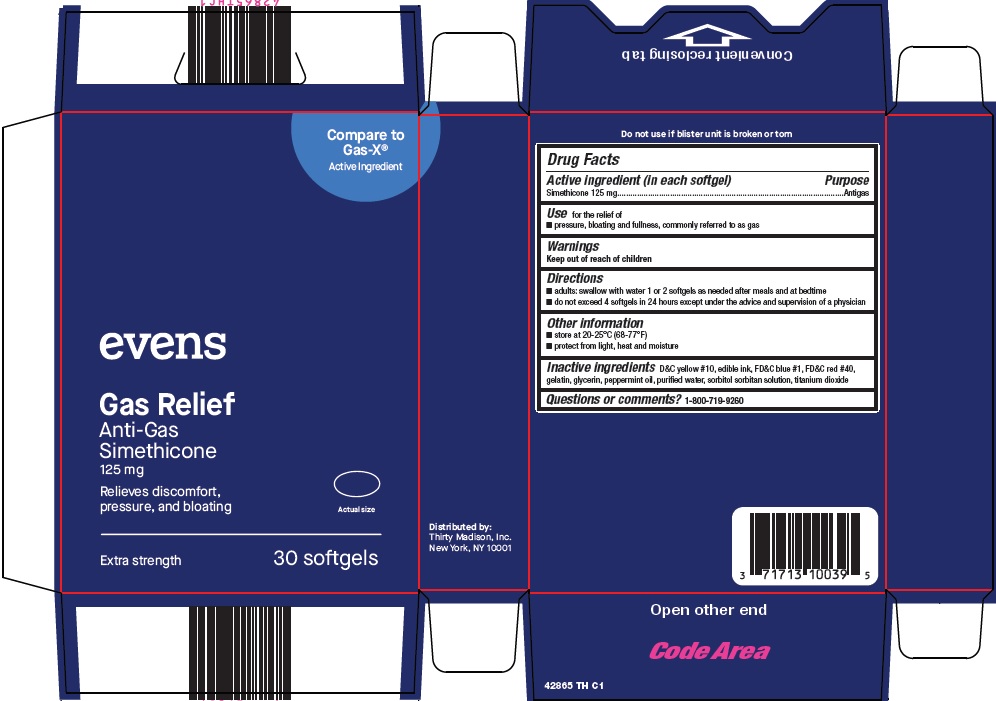
Thirty Madison Inc Gas Relief Drug Facts
gas relief by
Drug Labeling and Warnings
gas relief by is a Otc medication manufactured, distributed, or labeled by THIRTY MADISON INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GAS RELIEF- simethicone capsule, liquid filled
THIRTY MADISON INC
----------
Thirty Madison Inc Gas Relief Drug Facts
Directions
- adults: swallow with water 1 or 2 softgels as needed after meals and at bedtime
- do not exceed 4 softgels in 24 hours except under the advice and supervision of a physician
| GAS RELIEF
simethicone capsule, liquid filled |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - THIRTY MADISON INC (080774087) |
Revised: 10/2024
Document Id: c2f1b840-ef8a-4257-903b-0702f7cf9321
Set id: e520eea6-4c61-4e46-9ed8-56b49bbe018d
Version: 2
Effective Time: 20241015
Trademark Results [gas relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GAS RELIEF 85443589 not registered Dead/Abandoned |
Traditional Medicinals 2011-10-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.