SLEEP AID- diphenhydramine hydrochloride capsule, liquid filled
Sleep Aid by
Drug Labeling and Warnings
Sleep Aid by is a Otc medication manufactured, distributed, or labeled by CVS PHARMACY, INC., Humanwell PuraCap Pharmaceutical (Wuhan) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema, or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- heart disease
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers or any other sleep-aid
When using this product
- avoid alcoholic beverages and other drugs that cause drowsiness
- drowsiness will occur
- be careful when driving a motor vehicle or operating machinery
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
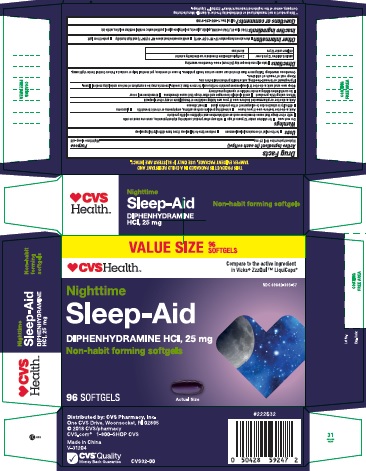
PRINCIPAL DISPLAY PANEL - Carton Label
Nighttime Sleep-Aid 96 SOFTGELS
NDC: 69842-393-57
*Compare to the active ingredient in Vicks® ZzzQuil® LiquiCaps®
-
INGREDIENTS AND APPEARANCE
SLEEP AID
diphenhydramine hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-393 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SORBITAN (UNII: 6O92ICV9RU) Product Characteristics Color purple (clear) Score no score Shape capsule (oblong) Size 15mm Flavor Imprint Code PC11 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-393-57 8 in 1 CARTON 09/25/2017 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part338 09/25/2017 Labeler - CVS PHARMACY, INC. (062312574) Establishment Name Address ID/FEI Business Operations Humanwell PuraCap Pharmaceutical (Wuhan) Co., Ltd 421293287 manufacture(69842-393) , analysis(69842-393)
Trademark Results [Sleep Aid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SLEEP AID 97858312 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-03-27 |
 SLEEP AID 97847521 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-03-20 |
 SLEEP AID 97765561 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-01-24 |
 SLEEP AID 88743606 not registered Live/Pending |
Plant Therapy LLC 2019-12-31 |
 SLEEP AID 78688138 3120450 Dead/Cancelled |
T. Harmon Inc. 2005-08-08 |
 SLEEP AID 77928191 not registered Dead/Abandoned |
EVEREST NUTRITION CORP 2010-02-04 |
 SLEEP AID 77448243 not registered Dead/Abandoned |
HEALCEUTICALS, LLC 2008-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.