ROBAFEN AC- guaifenesin and codeine phosphate syrup
Robafen AC by
Drug Labeling and Warnings
Robafen AC by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use
- in adults and children who have a chronic pulmonary disease or shortness of breath, or children who are taking other drugs, unless directed by a doctor.
- In children following a tonsillectomy and/or adenoidectomy as this could cause severe respiratory distress.
Ask a doctor before use if you have
- a cough with too much phlegm (mucus)
- a persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use if you are taking sedatives, tranquilizers and drugs used for depression, especially monoamine oxidase inhibitors (MAOIs). These combinations may cause greater sedation (drowsiness) than is caused by the product used alone.
-
Directions
- take every 4 hours
- do not exceed 6 doses in 24 hours
- giving a higher dose than recommended by a doctor can result in serious side effects for a child. Do not exceed recommended dosage.
- not intended for use in children under 6 years of age
- TSP = teaspoonful
Age Dose adults and children 12 years and over 2 TSP children 6 to under 12 years of age 1 TSP children under 6 years of age do not use -
Other information
- each (5 mL) teaspoon contains : 4 mg sodium
- Tamper evident: Do not use this product if inner foil seal over the mouth of the bottle is torn, broken or missing.
- Keep container closed and store away from heat. DO NOT REFRIGERATE.
- Store at room temperature 20°-25°C (68°-77°F)
- Dispense in a tight, light-resistant container as defined in the USP.
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC: 0904-6479-16
Robafen AC™
Oral Solution CV(GUAIFENESIN AND CODEINE
PHOSPHATE ORAL SOLUTION USP)
ANTITUSSIVE EXPECTORANT
SUGAR FREEUnder Federal law Robafen AC is
available without a prescription.
Certain State laws may differ.BULK CONTAINER - NOT FOR HOUSEHOLD USE.
TAMPER-EVIDENT
DO NOT REFRIGERATEManufactured by:
Bio-Pharm, Inc. Levittown, PA 19057
Distributed by: MAJOR ® PHARMACEUTICALS
17177 N Laurel Park Dr., Suite 233, Livonia, MI 48152
35-8045-16
REV 08/17 M-64ONE PINT (473 mL)
MAJOR ®
PHARMACEUTICALS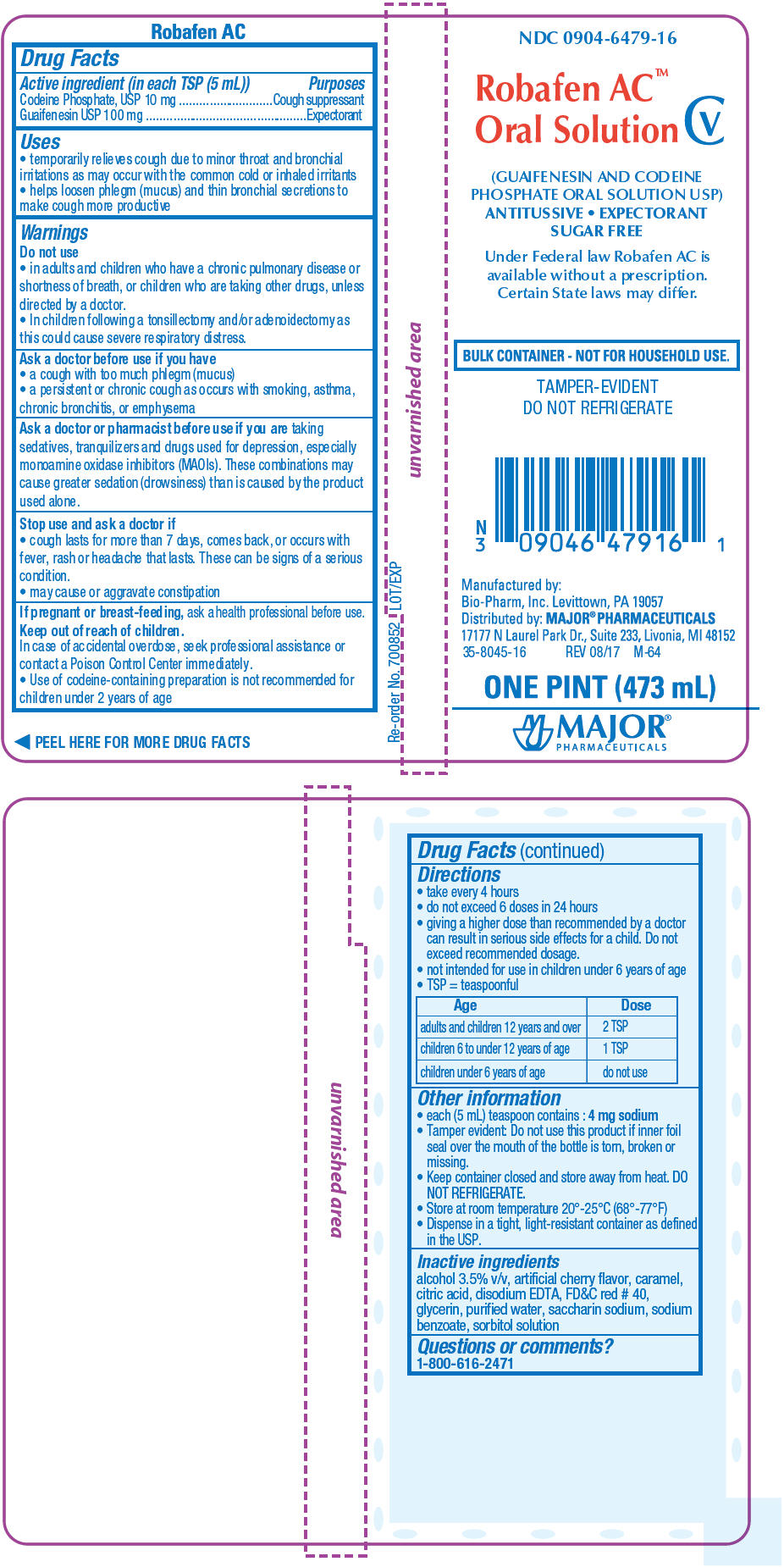
-
INGREDIENTS AND APPEARANCE
ROBAFEN AC
guaifenesin and codeine phosphate syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6479 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CHERRY (UNII: BUC5I9595W) CARAMEL (UNII: T9D99G2B1R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color brown Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6479-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/14/2017 10/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/14/2017 10/31/2020 Labeler - Major Pharmaceuticals (191427277)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.