Major Pharmaceuticals Robafen® Drug Facts
robafen by
Drug Labeling and Warnings
robafen by is a Otc medication manufactured, distributed, or labeled by Preferred Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ROBAFEN- dextromethorphan hbr, guaifenesin solution
Preferred Pharmaceuticals, Inc.
----------
Major Pharmaceuticals Robafen® Drug Facts
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- keep dosing cup with product
- mL = milliliter
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
10 mL every 4 hours |
|
children under 12 years |
do not use |
Inactive ingredients
anhydrous citric acid, FD&C red no. 40, flavor, glycerin, high fructose corn syrup, menthol, propylene glycol, purified water, sodium benzoate, sodium citrate, sucralose
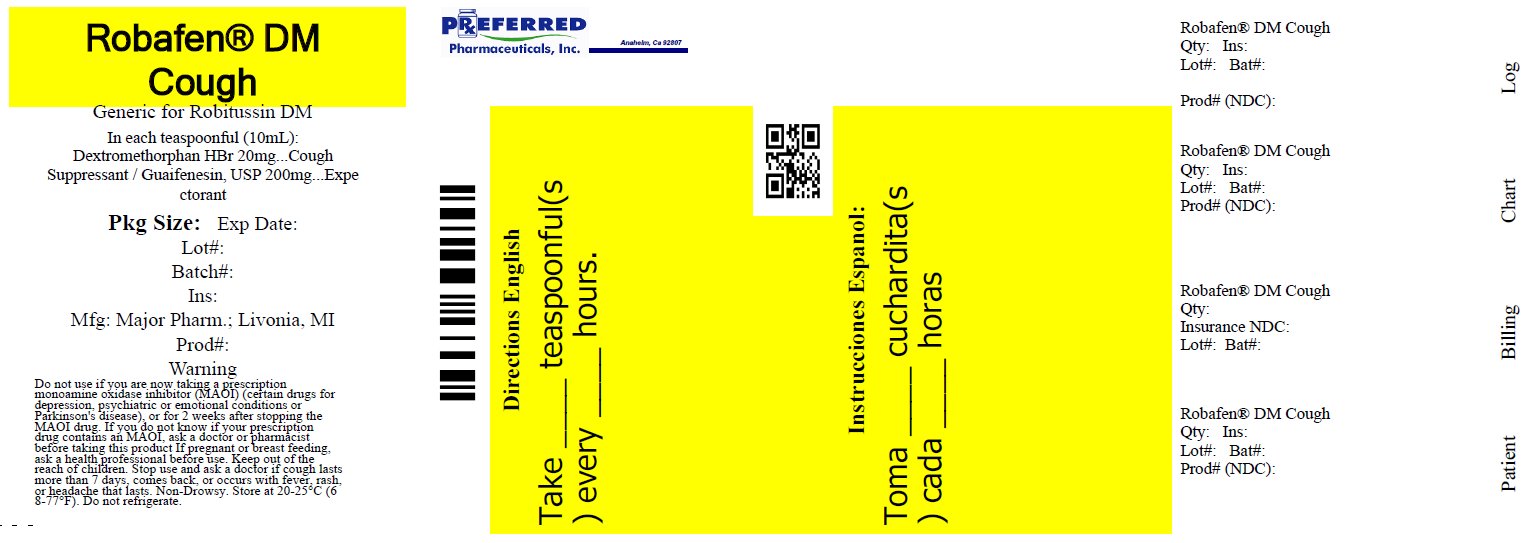
Principal Display Panel
NDC: 68788-7663-02
ROBAFEN®
DM COUGH
COUGH SUPPRESSANT (Dextromethorphan HBr)
EXPECTORANT (Guaifenesin)
PEAK COLD
Relieves:
Cough
Mucus
Non-Drowsy
COMPARE TO the active ingredients of ROBITUSSIN® COUGH + CHEST CONGESTION DM
FOR ADULTS 12 and Over
8 FL. OZ. (237 mL)
Relabeled by Preferred Pharmaceuticals, Inc.
| ROBAFEN
dextromethorphan hbr, guaifenesin solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals, Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals, Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals, Inc. | 791119022 | RELABEL(68788-7663) | |
Trademark Results [robafen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ROBAFEN 77766289 3742296 Live/Registered |
Harvard Drug Group, LLC 2009-06-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.