BEYOU Pink by Curaden AG / Trybol AG
BEYOU Pink by
Drug Labeling and Warnings
BEYOU Pink by is a Otc medication manufactured, distributed, or labeled by Curaden AG, Trybol AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BEYOU PINK- toothpasteВ paste, dentifriceВ
Curaden AG
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
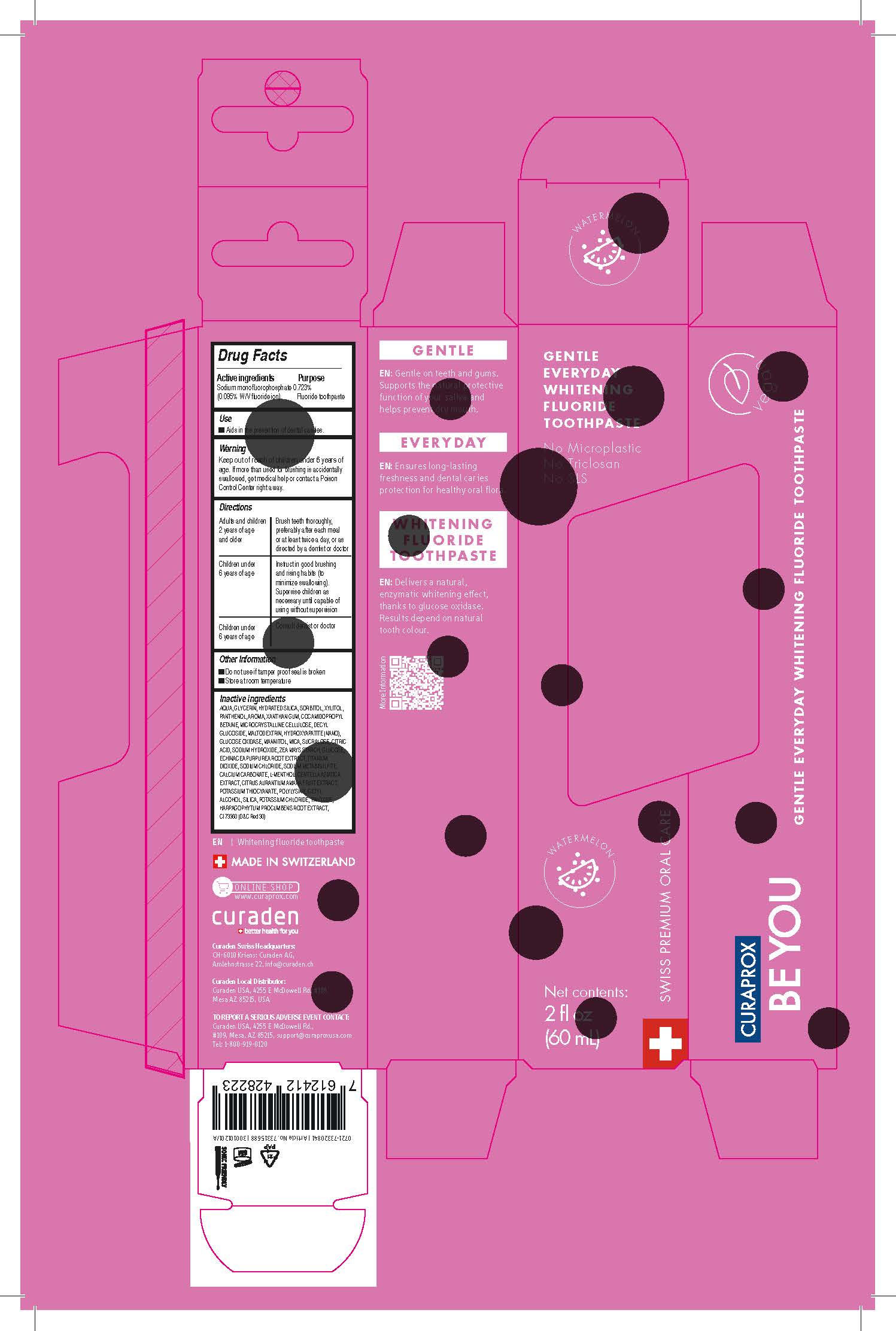
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.

 A pea-sized amount of toothpaste is enough.
A pea-sized amount of toothpaste is enough.
Be gentle when brushing and use a toothbrush with soft, high-density bristles.
Aqua, Glycerin, Hydrated silica, Sorbitol, Xylitol, Panthenol, Aroma, Xanthan gum, cocamidropropyl betaine, Microcrystalline cellulose, Decyl glucoside, Malodextrin, Hydroxyapatite (nano), Glucose oxidase, Mannitol, Mica, Sucralose, Citric acid, Sodium hydroxide, Zea mays starch, Glucose, Echinacea purpurea root extract, Titanium dioxide, Sodium chloride, D-limonene, Sodium metabisulfite, Calcium carbonate, L-methol, Centella asiatica extract, Citrus aurantium amara fruit extract, Potassium thiocyanate, Polylysine, Cetyl alcohol, Silica, Potassium chloride, Tin oxide, Harpagophytum procumbens root extract, CI 73360

| BEYOU PINKВ
toothpaste paste, dentifrice |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler -В Curaden AG (481145555) |
| Registrant -В Trybol AG (480305077) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Trybol AG | 480305077 | manufacture(71112-002) | |
В© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.