DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE- diltiazem hydrochloride capsule, extended release
Diltiazem Hydrochloride Extended-Release by
Drug Labeling and Warnings
Diltiazem Hydrochloride Extended-Release by is a Prescription medication manufactured, distributed, or labeled by Dr. Reddy's Labratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2, 3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride, (+)-cis-. The chemical structure is
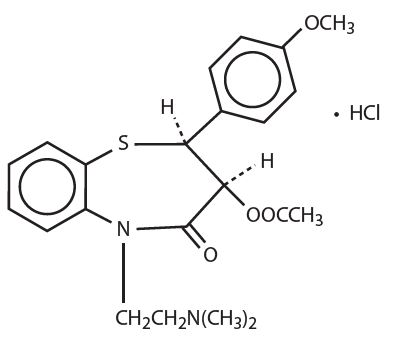
Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol, and chloroform. It has a molecular weight of 450.98. Each Diltiazem Hydrochloride Extended-Release Capsule, USP contains either 60 mg diltiazem hydrochloride (equivalent to 55.1 mg diltiazem), 90 mg diltiazem hydrochloride (equivalent to 82.7 mg diltiazem), or 120 mg diltiazem hydrochloride (equivalent to 110.3 mg diltiazem).
Also contains: sucrose, povidone, hypromellose, methacrylic acid copolymer, diethyl phthalate, FD&C Red No. 40, titanium dioxide, gelatin, black ink. The 90 mg also contains FD&C Red No. 3.
For oral administration.
FDA approved dissolution test specifications differ from USP.
-
CLINICAL PHARMACOLOGY
The therapeutic effects of diltiazem hydrochloride are believed to be related to its ability to inhibit the influx of calcium ions during membrane depolarization of cardiac and vascular smooth muscle.
Mechanism of Action
Diltiazem Hydrochloride Extended-Release Capsules produces its antihypertensive effect primarily by relaxation of vascular smooth muscle and the resultant decrease in peripheral vascular resistance. The magnitude of blood pressure reduction is related to the degree of hypertension; thus, hypertensive individuals experience an antihypertensive effect, whereas there is only a modest fall in blood pressure in normotensives.
Hemodynamic and Electrophysiological Effects
Like other calcium channel antagonists, diltiazem decreases sinoatrial and atrioventricular conduction in isolated tissues and has a negative inotropic effect in isolated preparations. In the intact animal, prolongation of the AH interval can be seen at higher doses.
In man, diltiazem prevents spontaneous and ergonovine-provoked coronary artery spasm. It causes a decrease in peripheral vascular resistance and a modest fall in blood pressure in normotensive individuals and, in exercise tolerance studies in patients with ischemic heart disease, reduces the heart rate-blood pressure product for any given workload. Studies to date, primarily in patients with good ventricular function, have not revealed evidence of a negative inotropic effect; cardiac output, ejection fraction, and left ventricular end diastolic pressure have not been affected. Increased heart failure has, however, been reported in occasional patients with preexisting impairment of ventricular function. There are as yet few data on the interaction of diltiazem and beta-blockers in patients with poor ventricular function. Resting heart rate is usually slightly reduced by diltiazem.
Diltiazem Hydrochloride Extended-Release Capsules produces antihypertensive effects both in the supine and standing positions. Postural hypotension is infrequently noted upon suddenly assuming an upright position. No reflex tachycardia is associated with the chronic antihypertensive effects. Diltiazem Hydrochloride Extended-Release Capsules decrease vascular resistance, increase cardiac output (by increasing stroke volume), and produce a slight decrease or no change in heart rate. During dynamic exercise, increases in diastolic pressure are inhibited, while maximum achievable systolic pressure is usually reduced. Heart rate at maximum exercise does not change or is slightly reduced. Chronic therapy with diltiazem hydrochloride produces no change or an increase in plasma catecholamines. No increased activity of the reninangiotensin-aldosterone axis has been observed. Diltiazem Hydrochloride Extended-Release Capsules antagonize the renal and peripheral effects of angiotensin II. Hypertensive animal models respond to diltiazem with reductions in blood pressure and increased urinary output and natriuresis without a change in urinary sodium/potassium ratio.
Intravenous diltiazem hydrochloride in doses of 20 mg prolongs AH conduction time and AV node functional and effective refractory periods by approximately 20%. In a study involving single oral doses of 300 mg of diltiazem hydrochloride in six normal volunteers, the average maximum PR prolongation was 14% with no instances of greater than first-degree AV block. Diltiazem-associated prolongation of the AH interval is not more pronounced in patients with first-degree heart block. In patients with sick sinus syndrome, diltiazem significantly prolongs sinus cycle length (up to 50% in some cases).
Chronic oral administration of diltiazem hydrochloride in doses of up to 360 mg/day has resulted in small increases in PR interval, and on occasion produces abnormal prolongation. (See WARNINGS)
Pharmacokinetics and Metabolism
Diltiazem is well absorbed from the gastrointestinal tract and is subject to an extensive first-pass effect, giving an absolute bioavailability (compared to intravenous administration) of about 40%. Diltiazem hydrochloride undergoes extensive metabolism in which 2% to 4% of the unchanged drug appears in the urine. In vitro binding studies show diltiazem hydrochloride is 70% to 80% bound to plasma proteins. Competitive in vitro ligand binding studies have also shown diltiazem hydrochloride binding is not altered by therapeutic concentrations of digoxin, hydrochlorothiazide, phenylbutazone, propranolol, salicylic acid, or warfarin. The plasma elimination half-life following single or multiple drug administration is approximately 3.0 to 4.5 hours. Desacetyl diltiazem is also present in the plasma at levels of 10% to 20% of the parent drug and is 25% to 50% as potent a coronary vasodilator as diltiazem. Minimum therapeutic plasma levels of diltiazem hydrochloride appear to be in the range of 50 - 200 ng/mL. There is a departure from linearity when dose strengths are increased; the half-life is slightly increased with dose. A study that compared patients with normal hepatic function to patients with cirrhosis found an increase in half-life and a 69% increase in bioavailability in the hepatically impaired patients. A single study in nine patients with severely impaired renal function showed no difference in the pharmacokinetic profile of diltiazem compared to patients with normal renal function.
Diltiazem Hydrochloride Extended-Release Capsules (Twice-a-Day Dosage)
A single 120 mg dose of the capsule results in detectable plasma levels within 2 to 3 hours and peak plasma levels at 6 to 11 hours. The apparent elimination half-life after single or multiple dosing is 5 to 7 hours. A departure from linearity similar to that observed with the diltiazem hydrochloride tablet is observed. As the dose of diltiazem hydrochloride extended-release capsules is increased from a daily dose of 120 mg (60 mg b.i.d.) to 240 mg (120 mg b.i.d.) daily, there is an increase in area-under-the-curve of 2.6 times. When the dose is increased from 240 mg to 360 mg daily, there is an increase in area-under-the-curve of 1.8 times. The average plasma levels of the capsule dosed twice daily at steady-state are equivalent to the tablet dosed four times daily when the same total daily dose is administered.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Diltiazem hydrochloride is contraindicated in (1) patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker, (2) patients with second- or third-degree AV block except in the presence of a functioning ventricular pacemaker, (3) patients with hypotension (less than 90 mm Hg systolic), (4) patients who have demonstrated hypersensitivity to the drug, and (5) patients with acute myocardial infarction and pulmonary congestion documented by X-ray on admission.
-
WARNINGS
Cardiac Conduction
Diltiazem hydrochloride prolongs AV node refractory periods without significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This effect may rarely result in abnormally slow heart rates (particularly in patients with sick sinus syndrome) or second- or third- degree AV block (9 of 2,111 patients or 0.43%). Concomitant use of diltiazem with beta-blockers or digitalis may result in additive effects on cardiac conduction. A patient with Prinzmetal's angina developed periods of asystole (2 to 5 seconds) after a single dose of 60 mg of diltiazem. (See ADVERSE REACTIONS.)
Congestive Heart Failure
Although diltiazem has a negative inotropic effect in isolated animal tissue preparations, hemodynamic studies in humans with normal ventricular function have not shown a reduction in cardiac index nor consistent negative effects on contractility (dp/dt). An acute study of oral diltiazem in patients with impaired ventricular function (ejection fraction 24% ± 6%) showed improvement in indices of ventricular function without significant decrease in contractile function (dp/dt). Experience with the use of diltiazem hydrochloride in combination with betablockers in patients with impaired ventricular function is limited. Caution should be exercised when using this combination.
Hypotension
Decreases in blood pressure associated with diltiazem hydrochloride therapy may occasionally result in symptomatic hypotension.
Acute Hepatic Injury
Mild elevations of transaminases with and without concomitant elevation in alkaline phosphatase and bilirubin have been observed in clinical studies. Such elevations were usually transient and frequently resolved even with continued diltiazem treatment. In rare instances, significant elevations in enzymes such as alkaline phosphatase, LDH, SGOT, SGPT, and other phenomena consistent with acute hepatic injury have been noted. These reactions tended to occur early after therapy initiation (1 to 8 weeks) and have been reversible upon discontinuation of drug therapy. The relationship to diltiazem hydrochloride is uncertain in some cases, but probable in some. (See PRECAUTIONS.)
-
PRECAUTIONS
General
Diltiazem hydrochloride is extensively metabolized by the liver and excreted by the kidneys and in bile. As with any drug given over prolonged periods, laboratory parameters of renal and hepatic function should be monitored at regular intervals. The drug should be used with caution in patients with impaired renal or hepatic function. In subacute and chronic dog and rat studies designed to produce toxicity, high doses of diltiazem were associated with hepatic damage. In special subacute hepatic studies, oral doses of 125 mg/kg and higher in rats were associated with histological changes in the liver which were reversible when the drug was discontinued. In dogs, doses of 20 mg/kg were also associated with hepatic changes; however, these changes were reversible with continued dosing.
Dermatological events (see ADVERSE REACTIONS) may be transient and may disappear despite continued use of diltiazem hydrochloride. However, skin eruptions progressing to erythema multiforme and/or exfoliative dermatitis have also been infrequently reported. Should a dermatologic reaction persist, the drug should be discontinued.
Drug Interactions
Due to the potential for additive effects, caution and careful titration are warranted in patients receiving diltiazem hydrochloride concomitantly with any agents known to affect cardiac contractility and/or conduction (see WARNINGS). Pharmacologic studies indicate that there may be additive effects in prolonging AV conduction when using beta-blockers or digitalis concomitantly with diltiazem hydrochloride (see WARNINGS).
As with all drugs, care should be exercised when treating patients with multiple medications. Diltiazem is both a substrate and an inhibitor of the cytochrome P-450 3A4 enzyme system. Other drugs that are specific substrates, inhibitors, or inducers of this enzyme system may have a significant impact on the efficacy and side effect profile of diltiazem. Patients taking other drugs that are substrates of CYP450 3A4, especially patients with renal and/or hepatic impairment, may require dosage adjustment when starting or stopping concomitantly administered diltiazem in order to maintain optimum therapeutic blood levels.
Anesthetics
The depression of cardiac contractility, conductivity, and automaticity as well as the vascular dilation associated with anesthetics may be potentiated by calcium channel blockers. When used concomitantly, anesthetics and calcium blockers should be titrated carefully.
Benzodiazepines
Studies showed that diltiazem increased the AUC of midazolam and triazolam by 3- to 4-fold and the Cmax by 2-fold, compared to placebo. The elimination half-life of midazolam and triazolam also increased (1.5- to 2.5-fold) during coadministration with diltiazem. These pharmacokinetic effects seen during diltiazem coadministration can result in increased clinical effects (e.g., prolonged sedation) of both midazolam and triazolam.
Beta-blockers
Controlled and uncontrolled domestic studies suggest that concomitant use of diltiazem hydrochloride and beta-blockers is usually well tolerated, but available data are not sufficient to predict the effects of concomitant treatment in patients with left ventricular dysfunction or cardiac conduction abnormalities.
Administration of diltiazem hydrochloride concomitantly with propranolol in five normal volunteers resulted in increased propranolol levels in all subjects and bioavailability of propranolol was increased approximately 50%. In vitro, propranolol appears to be displaced from its binding sites by diltiazem. If combination therapy is initiated or withdrawn in conjunction with propranolol, an adjustment in the propranolol dose may be warranted (see WARNINGS).
Buspirone
In nine healthy subjects, diltiazem significantly increased the mean buspirone AUC 5.5-fold and Cmax 4.1-fold compared to placebo. The T1/2 and Tmax of buspirone were not significantly affected by diltiazem. Enhanced effects and increased toxicity of buspirone may be possible during concomitant administration with diltiazem. Subsequent dose adjustments may be necessary during coadministration and should be based on clinical assessment.
Carbamazepine
Concomitant administration of diltiazem with carbamazepine has been reported to result in elevated serum levels of carbamazepine (40% to 72% increase), resulting in toxicity in some cases. Patients receiving these drugs concurrently should be monitored for a potential drug interaction.
Cimetidine
A study in six healthy volunteers has shown a significant increase in peak diltiazem plasma levels (58%) and area-under-the-curve (53%) after a 1-week course of cimetidine at 1,200 mg per day and a single dose of diltiazem 60 mg. Ranitidine produced smaller, nonsignificant increases. The effect may be mediated by cimetidine's known inhibition of hepatic cytochrome P-450, the enzyme system responsible for the first-pass metabolism of diltiazem. Patients currently receiving diltiazem therapy should be carefully monitored for a change in pharmacological effect when initiating and discontinuing therapy with cimetidine. An adjustment in the diltiazem dose may be warranted.
Clonidine
Sinus bradycardia resulting in hospitalization and pacemaker insertion has been reported in association with the use of clonidine concurrently with diltiazem. Monitor heart rate in patients receiving concomitant diltiazem and clonidine.
Cyclosporine
A pharmacokinetic interaction between diltiazem and cyclosporine has been observed during studies involving renal and cardiac transplant patients. In renal and cardiac transplant recipients, a reduction of cyclosporine dose ranging from 15% to 48% was necessary to maintain cyclosporine trough concentrations similar to those seen prior to the addition of diltiazem. If these agents are to be administered concurrently, cyclosporine concentrations should be monitored, especially when diltiazem therapy is initiated, adjusted or discontinued. The effect of cyclosporine on diltiazem plasma concentrations has not been evaluated.
Digitalis
Administration of diltiazem hydrochloride with digoxin in 24 healthy male subjects increased plasma digoxin concentrations approximately 20%. Another investigator found no increase in digoxin levels in 12 patients with coronary artery disease. Since there have been conflicting results regarding the effect of digoxin levels, it is recommended that digoxin levels be monitored when initiating, adjusting, and discontinuing diltiazem hydrochloride therapy to avoid possible over- or under-digitalization (see WARNINGS).
Ivabradine
Concurrent use of diltiazem increases exposure to ivabradine and may exacerbate bradycardia and conduction disturbances. Avoid concomitant use of ivabradine and diltiazem.
Quinidine
Diltiazem significantly increases the AUC (0 →∞) of quinidine by 51%, T1/2 by 36%, and decreases its CLoral by 33%. Monitoring for quinidine adverse effects may be warranted and the dose adjusted accordingly.
Rifampin
Coadministration of rifampin with diltiazem lowered the diltiazem plasma concentrations to undetectable levels. Coadministration of diltiazem with rifampin or any known CYP3A4 inducer should be avoided when possible, and alternative therapy considered.
Statins
Diltiazem is an inhibitor of CYP3A4 and has been shown to increase significantly the AUC of some statins. The risk of myopathy and rhabdomyolysis with statins metabolized by CYP3A4 may be increased with concomitant use of diltiazem. When possible, use a nonCYP3A4-metabolized statin together with diltiazem; otherwise, dose adjustments for both diltiazem and the statin should be considered along with close monitoring for signs and symptoms of any statin related adverse events.
In a healthy volunteer cross-over study (N=10), coadministration of a single 20 mg dose of simvastatin at the end of a 14 day regimen with 120 mg BID diltiazem Extended-Release resulted in a 5-fold increase in mean simvastatin AUC versus simvastatin alone. Subjects with increased average steady-state exposures of diltiazem showed a greater fold increase in simvastatin exposure. Computer-based simulations showed that at a daily dose of 480 mg of diltiazem, an 8- to 9-fold mean increase in simvastatin AUC can be expected. If coadministration of simvastatin with diltiazem is required, limit the daily doses of simvastatin to 10 mg and diltiazem to 240 mg.
In a ten-subject randomized, open label, 4-way cross over study, co-administration of diltiazem (120 mg bid, diltiazem SR for 2 weeks) with single 20 mg dose of lovastatin resulted in 3- to 4fold increase in mean lovastatin AUC and Cmax versus lovastatin alone. In the same study, there was no significant change in 20 mg single dose pravastatin AUC and Cmax during diltiazem coadministration. Diltiazem plasma levels were not significantly affected by lovastatin or pravastatin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 24-month study in rats and a 21-month study in mice showed no evidence of carcinogenicity. There was also no mutagenic response in in vitro bacterial tests. No intrinsic effect on fertility was observed in rats.
Pregnancy
Reproduction studies have been conducted in mice, rats, and rabbits. Administration of doses ranging from five to ten times greater (on a mg/kg basis) than the daily recommended therapeutic dose has resulted in embryo and fetal lethality. These doses, in some studies, have been reported to cause skeletal abnormalities. In the perinatal/postnatal studies, there was some reduction in early individual pup weights and survival rates. There was an increased incidence of stillbirths at doses of 20 times the human dose or greater.
There are no well controlled studies in pregnant women; therefore, use diltiazem hydrochloride in pregnant women only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Diltiazem is excreted in human milk. One report suggests that concentrations in breast milk may approximate serum levels. If use of diltiazem hydrochloride is deemed essential, an alternative method of infant feeding should be instituted.
Geriatric Use
Clinical studies of diltiazem did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Serious adverse reactions have been rare in studies carried out to date, but it should be recognized that patients with impaired ventricular function and cardiac conduction abnormalities have usually been excluded from these studies.
The adverse events described below represent events observed in clinical studies of hypertensive patients receiving either diltiazem hydrochloride tablets or Diltiazem Hydrochloride Extended-Release Capsules, as well as experiences observed in studies of angina and during marketing. The most common events in hypertension studies are shown in a table with rates in placebo patients shown for comparison. Less common events are listed by body system; these include any adverse reactions seen in angina studies that were not observed in hypertension studies. In all hypertensive patients studied (over 900), the most common adverse events were edema (9%), headache (8%), dizziness (6%), asthenia (5%), sinus bradycardia (3%), flushing (3%), and first degree AV block (3%). Only edema and perhaps bradycardia and dizziness were dose related. The most common events observed in clinical studies (over 2,100 patients) of angina patients and hypertensive patients receiving diltiazem hydrochloride tablets or Diltiazem Hydrochloride Extended-Release Capsules were (i.e., greater than 1%) edema (5.4%), headache (4.5%), dizziness (3.4%), asthenia (2.8%), first-degree AV block (1.8%), flushing (1.7%), nausea (1.6%), bradycardia (1.5%), and rash (1.5%).
Double Blind Placebo Controlled Hypertension Trials Adverse Diltiazem
N = 315
# pts (%)Placebo
N = 211
# pts (%)Headache 38 (12%) 17 (8%) AV block first degree 24 (7.6%) 4 (1.9%) Dizziness 22 (7%) 6 (2.8%) Edema 19 (6%) 2 (0.9%) Bradycardia 19 (6%) 3 (1.4%) ECG abnormality 13 (4.1%) 3 (1.4%) Asthenia 10 (3.2%) 1 (0.5%) Constipation 5 (1.6%) 2 (0.9%) Dyspepsia 4 (1.3%) 1 (0.5%) Nausea 4 (1.3%) 2 (0.9%) Palpitations 4 (1.3%) 2 (0.9%) Polyuria 4 (1.3%) 2 (0.9%) Somnolence 4 (1.3%) — Alk phos increase 3 (1%) 1 (0.5%) Hypotension 3 (1%) 1 (0.5%) Insomnia 3 (1%) 1 (0.5%) Rash 3 (1%) 1 (0.5%) AV block second degree 2 (0.6%) — In addition, the following events were reported infrequently (less than 1%) with Diltiazem Hydrochloride Extended-Release Capsules or diltiazem hydrochloride tablets or have been observed in angina or hypertension trials.
Cardiovascular: Angina, arrhythmia, second- or third-degree AV block (see Conduction Warning), bundle branch block, congestive heart failure, syncope, tachycardia, ventricular extrasystoles.
Nervous System: Abnormal dreams, amnesia, depression, gait abnormality, hallucinations, nervousness, paresthesia, personality change, tremor.
Gastrointestinal: Anorexia, diarrhea, dry mouth, dysgeusia, mild elevations of SGOT, SGPT, and LDH (see Hepatic Warnings), thirst, vomiting, weight increase.
Dermatological: Petechiae, photosensitivity, pruritus, urticaria.
Other: Amblyopia, CPK increase, dyspnea, epistaxis, eye irritation, hyperglycemia, hyperuricemia, impotence, muscle cramps, nasal congestion, nocturia, osteoarticular pain, sexual difficulties, tinnitus.
The following post-marketing events have been reported infrequently in patients receiving diltiazem hydrochloride: acute generalized exanthematous pustulosis, allergic reactions, alopecia, angioedema (including facial or periorbital edema), asystole, erythema multiforme (including Stevens-Johnson Syndrome, toxic epidermal necrolysis), extrapyramidal symptoms, gingival hyperplasia, hemolytic anemia, increased bleeding time, leukopenia, photosensitivity (including lichenoid keratosis and hyperpigmentation at sun-exposed skin areas), purpura, retinopathy, myopathy, and thrombocytopenia. There have been observed cases of a generalized rash, some characterized as leukocytoclastic vasculitis. In addition, events such as myocardial infarction have been observed which are not readily distinguishable from the natural history of the disease in these patients. A definitive cause and effect relationship between these events and diltiazem hydrochloride therapy cannot yet be established. Exfoliative dermatitis (proven by rechallenge) has also been reported.
To report SUSPECTED ADVERSE EVENTS, contact Dr. Reddy’s Laboratories Inc, at 1-888-375-3784 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch for voluntary reporting of adverse reactions.
-
OVERDOSAGE
The oral LD50's in mice and rats range from 415 to 740 mg/kg and from 560 to 810 mg/kg, respectively. The intravenous LD50's in these species were 60 and 38 mg/kg, respectively. The oral LD50 in dogs is considered to be in excess of 50 mg/kg, while lethality was seen in monkeys at 360 mg/kg.
The toxic dose in man is not known. Due to extensive metabolism, blood levels after a standard dose of diltiazem can vary over tenfold, limiting the usefulness of blood levels in overdose cases.
There have been reports of diltiazem overdose in doses ranging from 1 g to 18 g. Of cases with known outcome, most patients recovered and in cases with a fatal outcome, the majority involved multiple drug ingestion.
Events observed following diltiazem overdose included bradycardia, hypotension, heart block, and cardiac failure. Most reports of overdose described some supportive medical measure and/or drug treatment. Bradycardia frequently responded favorably to atropine, as did heart block, although cardiac pacing was also frequently utilized to treat heart block. Fluids and vasopressors were used to maintain blood pressure and in cases of cardiac failure inotropic agents were administered. In addition, some patients received treatment with ventilatory support, gastric lavage, activated charcoal, and/or intravenous calcium.
The effectiveness of intravenous calcium administration to reverse the pharmacological effects of diltiazem overdose has been inconsistent. In a few reported cases, overdose with calcium channel blockers associated with hypotension and bradycardia that was initially refractory to atropine became more responsive to atropine after the patients received intravenous calcium. In some cases, intravenous calcium has been administered (1 g calcium chloride or 3 g calcium gluconate) over 5 minutes and repeated every 10-20 minutes as necessary. Calcium gluconate has also been administered as a continuous infusion at a rate of 2 g per hour for 10 hours. Infusions of calcium for 24 hours or more may be required. Patients should be monitored for signs of hypercalcemia.
In the event of overdosage or exaggerated response, appropriate supportive measures should be employed in addition to gastrointestinal decontamination. Diltiazem does not appear to be removed by peritoneal or hemodialysis. Limited data suggest that plasmapheresis or charcoal hemoperfusion may hasten diltiazem elimination following overdose. Based on the known pharmacological effects of diltiazem and/or reported clinical experiences the following measures may be considered:
Bradycardia
Administer atropine (0.6 to 1 mg). If there is no response to vagal blockade, administer isoproterenol cautiously.
-
DOSAGE AND ADMINISTRATION
Dosages must be adjusted to each patient's needs, starting with 60 to 120 mg twice daily. Maximum antihypertensive effect is usually observed by 14 days of chronic therapy; therefore, dosage adjustments should be scheduled accordingly. Although individual patients may respond to lower doses, the usual optimum dosage range in clinical trials was 240 to 360 mg/day.
Diltiazem hydrochloride extended-release Capsules have an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride extended-release capsules or the concomitant antihypertensives may need to be adjusted when adding one to the other. See WARNINGS and PRECAUTIONS regarding use with beta-blockers.
-
HOW SUPPLIED
Diltiazem Hydrochloride Extended-Release Capsules, USP (Twice a Day Dosage) are available as follows:
Strength Quantity NDC Number Description 60 mg 100 75907-044-01 The 60 mg capsules are hard shell gelatin capsules with a coral cap and a white body filled with white to off-white pellets. The capsules are printed with 328 on the body. 90 mg 100 75907-045-01 The 90 mg capsules are hard shell gelatin capsules with a coral cap and a pink body filled with white to off-white pellets. The capsules are printed with 329 on the body. 120 mg 100 75907-046-01 The 120 mg capsules are hard shell gelatin capsules with a coral cap and a coral body filled with white to off-white pellets. The capsules are printed with 330 on the body. - SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 60 mg Capsule Bottle Label
NDC: 75907-044-01
Twice-a-Day Dosage
Diltiazem HCl
Extended-Release
Capsules, USP60 mg
Rx Only
100 CapsulesDr. Reddy's Laboratories, Inc

-
PRINCIPAL DISPLAY PANEL - 90 mg Capsule Bottle Label
NDC: 75907-045-01
Twice-a-Day Dosage
Diltiazem HCl
Extended-Release
Capsules, USP90 mg
Rx Only
100 CapsulesDr. Reddy's Laboratories, Inc

-
PRINCIPAL DISPLAY PANEL - 120 mg Capsule Bottle Label
NDC: 75907-046-01
Twice-a-Day Dosage
Diltiazem HCl
Extended-Release
Capsules, USP120 mg
Rx Only
100 CapsulesDr. Reddy's Laboratories, Inc

-
INGREDIENTS AND APPEARANCE
DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE
diltiazem hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75907-044 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength diltiazem hydrochloride (UNII: OLH94387TE) (diltiazem - UNII:EE92BBP03H) diltiazem hydrochloride 60 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) diethyl phthalate (UNII: UF064M00AF) FD&C Red No. 40 (UNII: WZB9127XOA) titanium dioxide (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) BLACK CURRANT (UNII: 9755T40D11) Product Characteristics Color ORANGE (coral) , WHITE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 328 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75907-044-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215775 09/10/2024 DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE
diltiazem hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75907-045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength diltiazem hydrochloride (UNII: OLH94387TE) (diltiazem - UNII:EE92BBP03H) diltiazem hydrochloride 90 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) diethyl phthalate (UNII: UF064M00AF) FD&C Red No. 40 (UNII: WZB9127XOA) titanium dioxide (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) BLACK CURRANT (UNII: 9755T40D11) FD&C Red No. 3 (UNII: PN2ZH5LOQY) Product Characteristics Color ORANGE (coral) , PINK Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 329 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75907-045-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215775 09/10/2024 DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE
diltiazem hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75907-046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength diltiazem hydrochloride (UNII: OLH94387TE) (diltiazem - UNII:EE92BBP03H) diltiazem hydrochloride 120 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) diethyl phthalate (UNII: UF064M00AF) FD&C Red No. 40 (UNII: WZB9127XOA) titanium dioxide (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) BLACK CURRANT (UNII: 9755T40D11) Product Characteristics Color ORANGE (coral) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 330 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75907-046-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215775 09/10/2024 Labeler - Dr. Reddy's Labratories Inc. (802315887)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.