Griselfulvin Oral Suspension, USP
Griseofulvin Oral Suspension by
Drug Labeling and Warnings
Griseofulvin Oral Suspension by is a Prescription medication manufactured, distributed, or labeled by TriRx Huntsville Pharmaceutical Services LLC, TriRx Huntsville Pharmaceutical Services. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
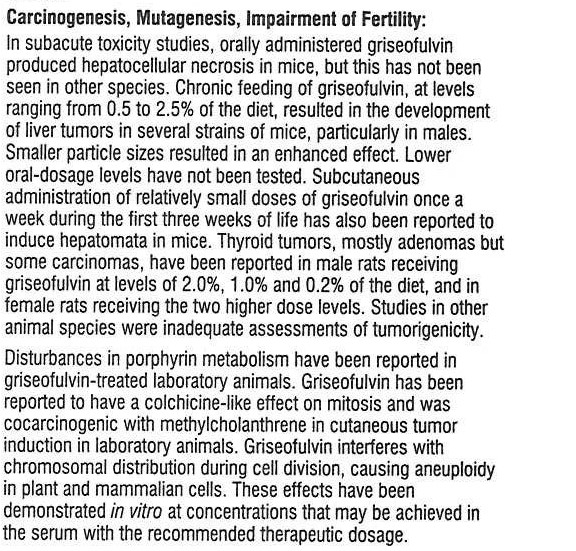
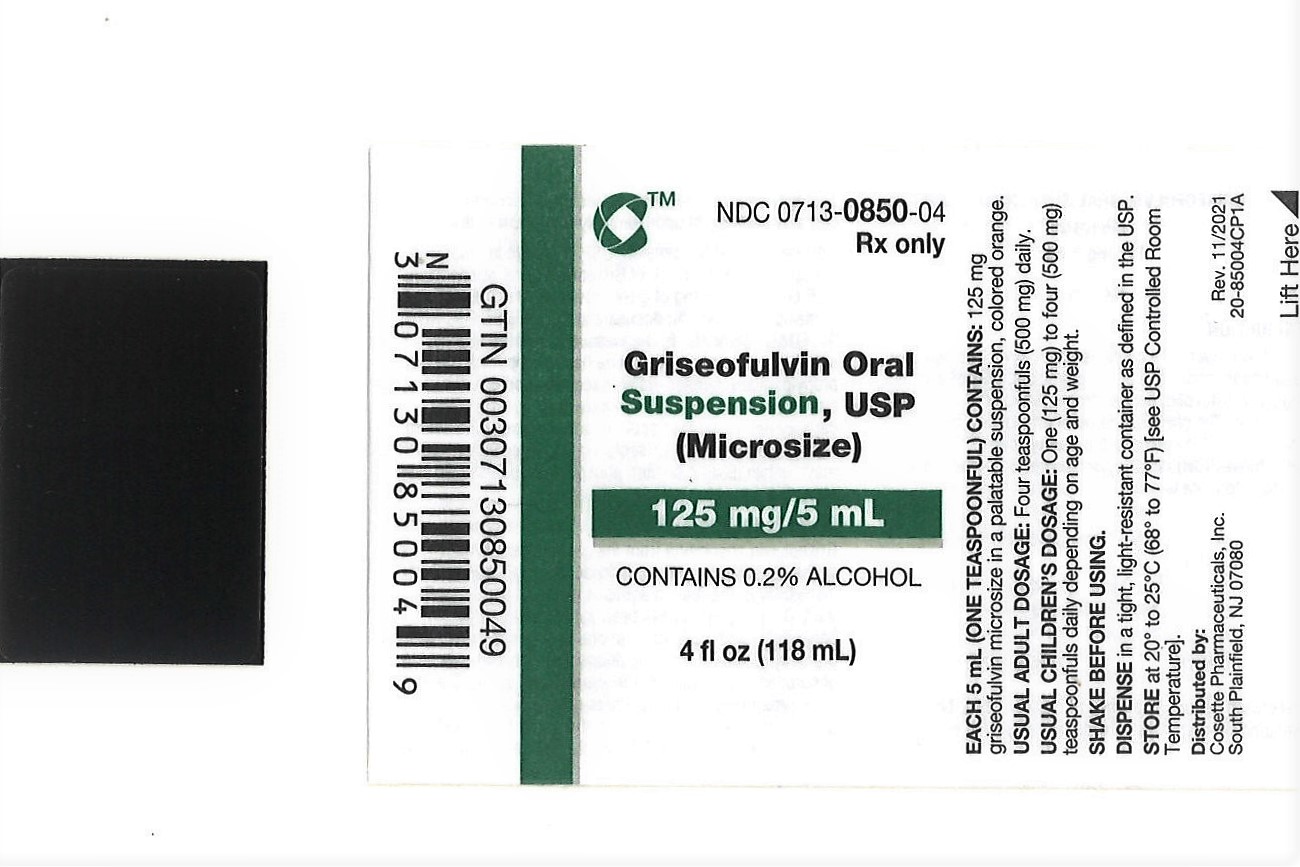
GRISEOFULVIN ORAL SUSPENSION- griseofulvin oral suspension suspension
TriRx Huntsville Pharmaceutical Services LLC
----------
Griselfulvin Oral Suspension, USP
| GRISEOFULVIN ORAL SUSPENSION
griseofulvin oral suspension suspension |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - TriRx Huntsville Pharmaceutical Services LLC (117090286) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TriRx Huntsville Pharmaceutical Services | 117090286 | manufacture(80432-056) | |
Revised: 1/2023
Document Id: f1ea506d-0062-7849-e053-2995a90a94ea
Set id: f1ea506d-0061-7849-e053-2995a90a94ea
Version: 1
Effective Time: 20230110
TriR
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.