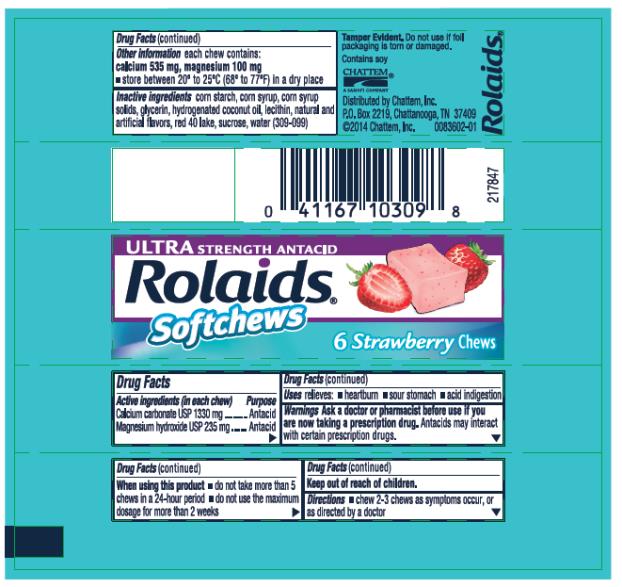
ROLAIDS ULTRA STRENGTH ANTACID STRAWBERRY- calcium carbonate and magnesium hydroxide tablet, chewable ROLAIDS ULTRA STRENGTH ANTACID ORANGE- calcium carbonate and magnesium hydroxide tablet, chewable
Rolaids Ultra Strength Antacid Orange by
Drug Labeling and Warnings
Rolaids Ultra Strength Antacid Orange by is a Otc medication manufactured, distributed, or labeled by Chattem, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Active ingredients
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROLAIDS ULTRA STRENGTH ANTACID STRAWBERRY
calcium carbonate and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41167-1031 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 1330 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 235 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CORN SYRUP (UNII: 9G5L16BK6N) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) FD&C RED NO. 40 (UNII: WZB9127XOA) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Product Characteristics Color PINK Score no score Shape RECTANGLE Size 20mm Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41167-1031-1 1 in 1 CARTON 12/01/2014 1 6 in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC: 41167-1031-2 2 in 1 PACKAGE; Type 0: Not a Combination Product 12/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part331 12/01/2014 ROLAIDS ULTRA STRENGTH ANTACID ORANGE
calcium carbonate and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41167-1030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 1330 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 235 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CORN SYRUP (UNII: 9G5L16BK6N) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE Score no score Shape RECTANGLE Size 20mm Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41167-1030-7 1 in 1 CARTON 12/01/2014 11/05/2020 1 6 in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part331 12/01/2014 11/05/2020 Labeler - Chattem, Inc. (003336013)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.