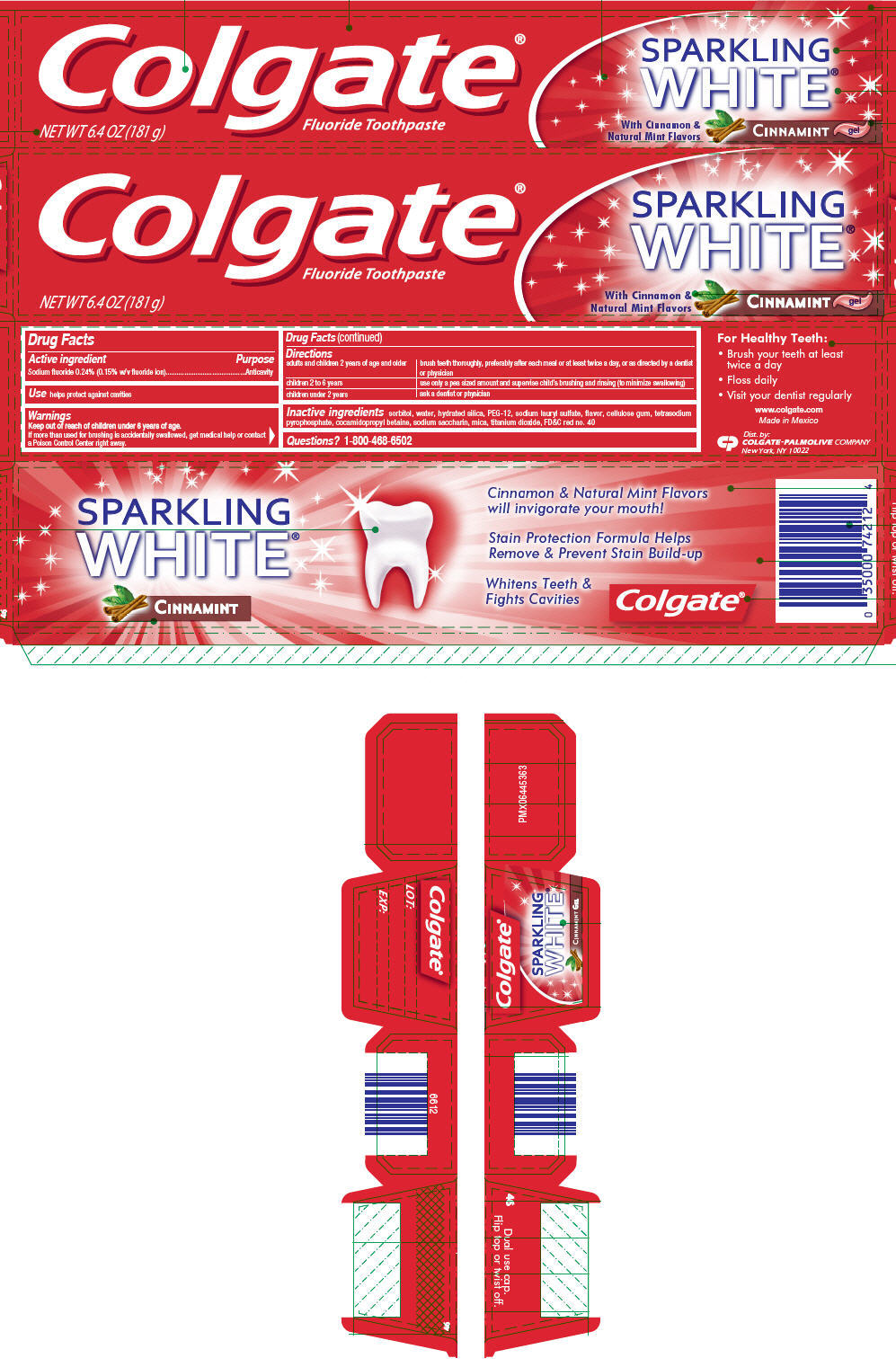
COLGATE® SPARKLING WHITE® CINNAMINT GEL
COLGATE SPARKLING WHITE CINNAMINT by
Drug Labeling and Warnings
COLGATE SPARKLING WHITE CINNAMINT by is a Otc medication manufactured, distributed, or labeled by Mission Hills S.A de C.V. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COLGATE SPARKLING WHITE CINNAMINT- sodium fluoride gel, dentifrice
Mission Hills S.A de C.V
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
COLGATE® SPARKLING WHITE® CINNAMINT GEL
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
| COLGATE SPARKLING WHITE CINNAMINT
sodium fluoride gel, dentifrice |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Mission Hills S.A de C.V (812312122) |
Revised: 4/2019
Document Id: b9313b31-95c7-48c0-9f8f-cc643e374a51
Set id: f604401a-1db6-41f8-9da3-995fa6faf5e5
Version: 3
Effective Time: 20190409
Mission Hills S.A de C.V