REVALOR XH- trenbolone acetate and estradiol implant
Revalor XH by
Drug Labeling and Warnings
Revalor XH by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp., Intervet GesmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Revalor®-XH is an extended-release implant. One dose (implant) contains 200 mg of trenbolone acetate and 20 mg estradiol in 6 coated and 4 uncoated pellets, each containing 20 mg trenbolone acetate and 2 mg estradiol.
The small yellow pellets are coated with a polymer to provide extended release of the active ingredients. One cartridge contains 10 doses.
Manufactured by a non-sterilizing process.
-
INDICATIONS FOR USE:
For increased rate of weight gain and improved feed efficiency for up to 200 days after implantation in beef heifers fed in confinement for slaughter.
Do not use in calves to be processed for veal. Effectiveness and animal safety in veal calves have not been established.
Not approved for repeated implantation (reimplantation) with this or any other cattle ear implant during the production phase(s) identified on labeling [beef heifers fed in confinement for slaughter] unless otherwise indicated on labeling because safety and effectiveness have not been evaluated.
Not to be used in animals intended for subsequent breeding, or in lactating dairy cows.
NOTE:
Studies have demonstrated that the administration of Revalor®-XH can result in decreased marbling scores when compared to non-implanted heifers.
-
DIRECTIONS
The cartridge is designed to be used with a special implanting tool. The special implanting tool is available from Intervet Inc. Ten doses (implants) are in each cartridge.
The implant is placed under the skin on the posterior aspect of the ear (see Site of Implantation and Method of Use sections).
With the animal suitably restrained, the skin on the outer surface of the ear should be cleaned. The implant is then administered by the method shown in the diagram below
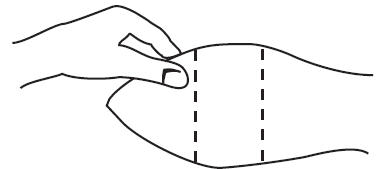
Fig. 1: Ear of Bovine Ready for Implantation
-
SITE OF IMPLANTATION
After appropriately restraining the animal to allow access to the ear, cleanse the skin at the implant needle puncture site. It is subcutaneous between the skin and cartilage on the back side of the ear and below the midline of the ear. The implant must not be placed closer to the head than the edge of the cartilage ring farthest from the head. The location of insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site. Care should be taken to avoid injuring the major blood vessels or cartilage of the ear.
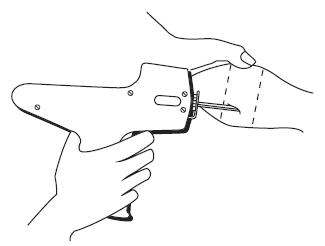
Fig. 2: Rear View of the Bovine Ear Showing the Site for Insertion of the Implanter needle.
-
METHOD OF USE
- Do not remove the cap of the cartridge containing the implants.
- Place the cartridge (D) with the capped end to the front into slot at the top of the implanter magazine marked (A) on the diagram.
- Gently push the cartridge into the slot until it clicks into place.
- The implanter is then ready for use.
- Take the ear of the animal firmly with the free hand in the manner shown in Fig. 1. Then insert the needle into the subcutaneous tissue at the point indicated in Fig. 2.
- After inserting the needle to its full extent, squeeze the trigger (E) gradually. Allow the pellets of the implant to be deposited in a single row.
- Withdraw the implanter. This will advance the cartridge one groove in the magazine and the next implant is now ready for use.
- When all the implants have been administered, the cartridge will discharge out the bottom of the magazine and may be replaced by a new one.
- To change the needle, loosen the needle locking nut labeled (F) in Fig. 3 and replace the needle. Tighten the nut finger tight and the implanter is ready for use.
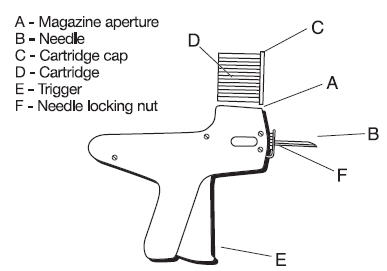
Fig. 3: Diagram of the Implanter and Cartridge.
-
WITHDRAWAL PERIODS AND RESIDUE WARNINGS:
No withdrawal period is required when used according to labeling.
Do not use in calves to be processed for veal. A withdrawal period has not been established for this product in pre-ruminating calves.
Do not use in lactating dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
Administer implant subcutaneously in the ear only. Any other location is in violation of Federal Law. Do not attempt salvage of implanted site for human or animal food.
-
USER SAFETY WARNINGS
Not for Use in Humans. Keep this and all drugs out of the reach of children.
To report suspected adverse drug experiences and/or to obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, call MERCK Animal Health at 1-800-211-3573.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or at http://www.fda.gov/AnimalVeterinary/SafetyHealth
-
STORAGE CONDITIONS
Store unopened product at or below 25°C (77°F). Avoid excessive heat and humidity. Use product before the expiration date printed on the labeling and on the cartridge pouch. Opened cartridges may be stored in the foil pouch protected from light in the refrigerator (2-8°C/36-47°F) for up to 6 months.
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
Made in Austria by:
Intervet GesmbHDistributed by:
Intervet Inc (d/b/a Merck Animal Health)
Madison, NJ 07940Restricted Drug (California) – use only as directed.
NADA 141-269, Approved by FDA
For Patent information:
http://www.merck.com/product/patent/home.htmlRevalor is a registered trademark of Intervet
Inc. or an affiliate. - PRINCIPAL DISPLAY PANEL - 10 x 10 Cartridge Implant Carton
-
INGREDIENTS AND APPEARANCE
REVALOR XH
trenbolone acetate and estradiol implantProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 57926-027 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trenbolone acetate (UNII: RUD5Y4SV0S) (Trenbolone - UNII:P53R4420TR) Trenbolone acetate 200 mg Estradiol (UNII: 4TI98Z838E) (Estradiol - UNII:4TI98Z838E) Estradiol 20 mg Inactive Ingredients Ingredient Name Strength Magnesium Stearate (UNII: 70097M6I30) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57926-027-01 10 in 1 CARTON 1 1 in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141269 12/15/2017 Labeler - Merck Sharp & Dohme Corp (001317601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
