TUSSIN DM COUGH SUPPRESSANT/EXPECTORANT- dextromethorphan hydrobromide, guaifenesin liquid
Tussin DM by
Drug Labeling and Warnings
Tussin DM by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LLC, AptaPharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
Do not use
- in a child under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding
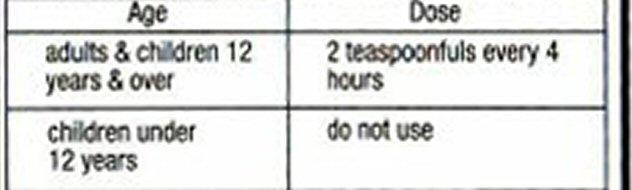
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Product Label
NDC: 68016-855-56
*COMPARE TO THE ACTIVE INGREDIENTS IN ROBITUSSIN® PEAK COLD COUGH and CHEST CONGESTION DMPremier Value®
Tussin DM
Dextromethorphan HBr
Guaifenesin
COUGH SUPPRESSANT /
EXPECTORANTNON-DROWSY
Helps to Loosen
Chest Congestion
Cough Formula
for ages 12 and over
8 FL OZ (237 mL)INDEPENDENTLY TESTED SATISFACTION GUARANTEED
DO NOT USE IF IMPRINTED SHRINK BAND IS MISSING OR BROKEN
*This product is not manufactured or distributed by Pfizer, owner of the registered trademark Robitussin® Peak Cold.
DISTRIBUTED BY:
CHAIN DRUG CONSORTIUM
3301 NW BOCA RATON BLVD
SUITE 101, BOCA RATON, FL 33431LR-018
LOT: EXP:

-
INGREDIENTS AND APPEARANCE
TUSSIN DM COUGH SUPPRESSANT/EXPECTORANT
dextromethorphan hydrobromide, guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-855 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-855-56 237 mL in 1 BOTTLE 2 NDC: 68016-855-57 354 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/01/2012 Labeler - Chain Drug Consortium, LLC (101668460) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(68016-855)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.