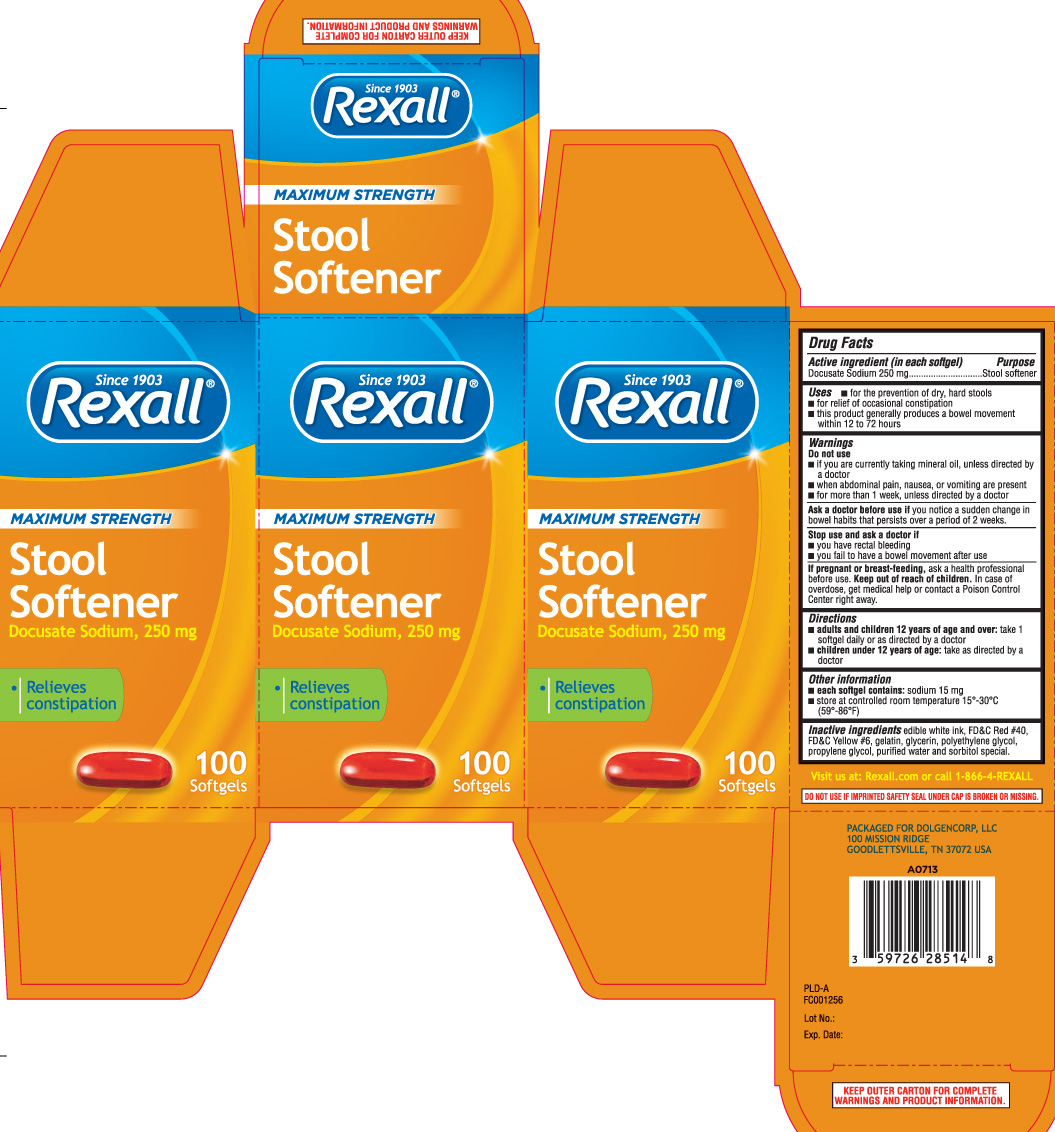
Stool Softener by Dolgencorp, Inc. (DOLLAR GENERAL & REXALL) / P & L Development, LLC Drug Facts
Stool Softener by
Drug Labeling and Warnings
Stool Softener by is a Otc medication manufactured, distributed, or labeled by Dolgencorp, Inc. (DOLLAR GENERAL & REXALL), P & L Development, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
STOOL SOFTENER MAXIMUM STRENGTH- docusate sodium capsule, liquid filled
Dolgencorp, Inc. (DOLLAR GENERAL & REXALL)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- for the prevention of dry, hard stools
- for relief of occasional constipation
- this product generally produces a bowel movement within 12 to 72 hours
Warnings
Do not use
- if you are currently taking mineral oil, unless directed by a doctor
- when abdominal pain, nausea, or vomiting are present
- for more than 1 week, unless directed by a doctor
Directions
- adults and children 12 years of age and over: take 1 softgel daily or as directed by a doctor
- children under 12 years of age: take as directed by a doctor
Other information
- each softgel contains: sodium 15 mg
- store at controlled room temperature 15º-30ºC (59º-86ºF)
Inactive ingredients
edible white ink, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol special
Principal Display Panel
Maximum Strength
Stool Softener
Docusate Sodium, 250 mg
- Relieves constipation
Softgels
Visit us at: Rexall.com or call 1-866-4-REXALL
PACKAGED FOR DOLGENCORP, LLC
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072 USA
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| STOOL SOFTENER
MAXIMUM STRENGTH
docusate sodium capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dolgencorp, Inc. (DOLLAR GENERAL & REXALL) (068331990) |
| Registrant - P & L Development, LLC (800014821) |