Children's Pain & Fever Acetaminophen Oral Suspension
Childrens Pain and Fever Acetaminophen by
Drug Labeling and Warnings
Childrens Pain and Fever Acetaminophen by is a Otc medication manufactured, distributed, or labeled by Rugby. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CHILDRENS PAIN AND FEVER ACETAMINOPHEN- acetaminophen suspension
Rugby
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Children's Pain & Fever Acetaminophen Oral Suspension
Uses
temporarily reduces fever and relieves minor aches and pains caused by
- headache
- common cold
- flu
- toothache
- sore throat
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert
acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
Sore throat warning
If sore throat is severe, lasts for more than 2 days or is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Do not use with any other drug containing acetaminophen (prescription or nonprescription.) If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
- this product does not contain directions or complete warnings for adult use
- shake well before using
- if possible, use weight to dose; otherwise use age
- use only with enclosed syringe. Do not use with any other dosing device
- push air out of syringe. Firmly push syringe into bottle opening.
- turn bottle upside-down and pull syringe to the correct dose
- dispense liquid slowly into child's mouth, towards inner cheek
- give dose every 4 hours while symptoms last
- do not give more than 5 doses in 24 hours
24-35 lbs (2-3 years): 5 mL
Under 24 lbs (under 2 years): ask a doctor
Inactive ingredients
Artificial flavor, citric acid anhydrous, corn syrup, FD&C Red #40, glycerin, microcrystalline cellulose and carboxymethylcellulose sodium, propylene glycol, purified water, sodium benzoate, sorbitol solution, sucralose, xanthan gum
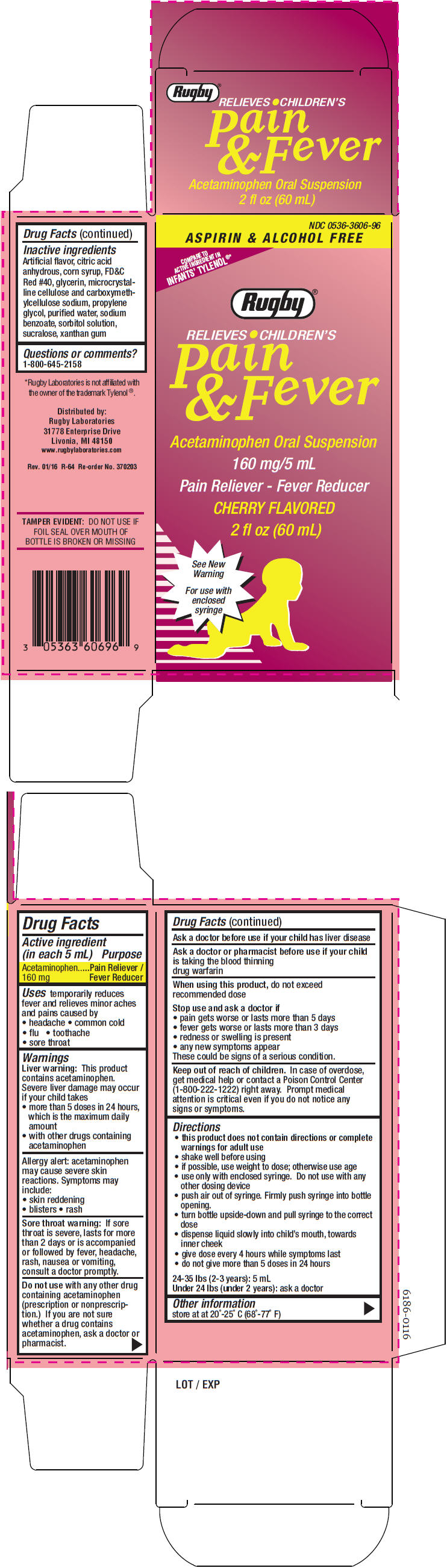
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Carton
NDC: 0536-3606-96
ASPIRIN & ALCOHOL FREE
COMPARE TO
ACTIVE INGREDIENT IN
INFANTS' TYLENOL®*
Rugby®
RELIEVES CHILDREN'S
Pain
& Fever
Acetaminophen Oral Suspension
160 mg/5 mL
Pain Reliever - Fever Reducer
CHERRY FLAVORED
2 fl oz (60 mL)
See New
Warning
For use with
enclosed
syringe
| CHILDRENS PAIN AND FEVER ACETAMINOPHEN
acetaminophen suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rugby (079246066) |