Cross-Fuse® II PEEK IBF System 38-2265-17
GUDID 00191083042275
INTERBODY FUSION DEVICE
Pioneer Surgical Technology, Inc.
Polymeric spinal interbody fusion cage| Primary Device ID | 00191083042275 |
| NIH Device Record Key | 46861d76-d16e-4b21-ba14-c16c1a1d1f9f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Cross-Fuse® II PEEK IBF System |
| Version Model Number | 38-2265-17 |
| Catalog Number | 38-2265-17 |
| Company DUNS | 793384496 |
| Company Name | Pioneer Surgical Technology, Inc. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com | |
| Phone | +1(906)226-9909 |
| labeling@resolvesurg.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00191083042275 [Primary] |
| GS1 | 00846468009281 [Previous] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K170643
FDA Product Code
| ODP | Intervertebral fusion device with bone graft, cervical |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-03-10 |
| Device Publish Date | 2022-03-02 |
On-Brand Devices [Cross-Fuse® II PEEK IBF System]
| 00191083033761 | Sterilization Case |
| 00191083033754 | Sterilization Case |
| 00191083033679 | TRIAL SPACER |
| 00191083033662 | TRIAL SPACER |
| 00191083033631 | TRIAL SPACER |
| 00191083033624 | TRIAL SPACER |
| 00191083033617 | TRIAL SPACER |
| 00191083033600 | TRIAL SPACER |
| 00191083033570 | TRIAL SPACER |
| 00191083033525 | TRIAL SPACER |
| 00191083033488 | TRIAL SPACER |
| 00191083033440 | TRIAL SPACER |
| 00191083033396 | TRIAL SPACER |
| 00191083033358 | TAMP |
| 00191083033341 | SLIDER |
| 00191083033334 | SLIDER |
| 00191083033327 | SLIDER |
| 00191083033310 | SLIDER |
| 00191083033303 | SLIDER |
| 00191083033273 | Inserter |
| 00191083033242 | INSERTER |
| 00191083033228 | INSERTER |
| 00191083033167 | L4/L5 TRIAL SPACER, RIGHT |
| 00191083033150 | L4/L5 TRIAL SPACER, LEFT |
| 00191083033143 | L4/L5 TRIAL SPACER, RIGHT |
| 00191083033136 | L4/L5 TRIAL SPACER, LEFT |
| 00191083033129 | L4/L5 TRIAL SPACER, RIGHT |
| 00191083033112 | L4/L5 TRIAL SPACER, LEFT |
| 00191083033105 | L4/L5 TRIAL SPACER, RIGHT |
| 00191083033099 | L4/L5 TRIAL SPACER, LEFT |
| 00191083033082 | L4/L5 TRIAL SPACER, RIGHT |
| 00191083033075 | L4/L5 TRIAL SPACER, LEFT |
| 00191083032788 | L4/L5 TRIAL SPACER |
| 00191083032771 | L4/L5 TRIAL SPACER |
| 00191083032764 | L4/L5 TRIAL SPACER |
| 00191083032733 | L4/L5 TRIAL SPACER |
| 00191083032696 | L4/L5 TRIAL SPACER |
| 00191083032689 | L4/L5 TRIAL SPACER |
| 00191083032672 | L4/L5 TRIAL SPACER |
| 00191083032641 | L4/L5 TRIAL SPACER |
| 00191083032597 | L4/L5 TRIAL SPACER |
| 00191083032542 | ANGLED INSERTER |
| 00191083043272 | INTERBODY FUSION DEVICE |
| 00191083043265 | INTERBODY FUSION DEVICE |
| 00191083042695 | INTERBODY FUSION DEVICE |
| 00191083042688 | INTERBODY FUSION DEVICE |
| 00191083042671 | INTERBODY FUSION DEVICE |
| 00191083042664 | INTERBODY FUSION DEVICE |
| 00191083042657 | INTERBODY FUSION DEVICE |
| 00191083042640 | INTERBODY FUSION DEVICE |
Trademark Results [Cross-Fuse]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
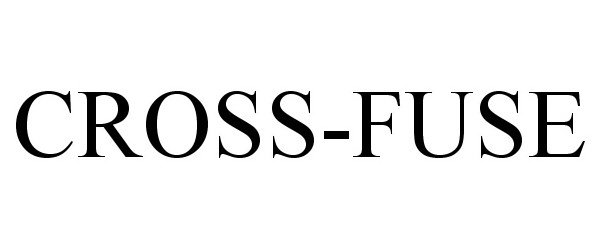 CROSS-FUSE 77284786 3544634 Live/Registered |
Pioneer Surgical Technology, Inc. 2007-09-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.