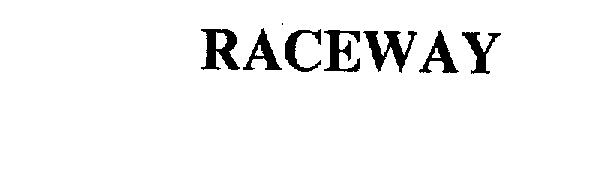Raceway 701067604
GUDID 00607567206496
DATASCOPE CORP.
Cardiopulmonary bypass system tubing set| Primary Device ID | 00607567206496 |
| NIH Device Record Key | 1dd68f94-7de5-4aed-a1cb-58d796e84e42 |
| Commercial Distribution Discontinuation | 2019-01-15 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Raceway |
| Version Model Number | BEQ-T 22707 |
| Catalog Number | 701067604 |
| Company DUNS | 001660786 |
| Company Name | DATASCOPE CORP. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | true |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com | |
| Phone | 18886278383 |
| cpsurgery@maquet.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 10 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00607567206496 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K080592
FDA Product Code
| DWE | Tubing, Pump, Cardiopulmonary Bypass |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
[00607567206496]
Ethylene Oxide
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-12-02 |
| Device Publish Date | 2015-11-21 |
Devices Manufactured by DATASCOPE CORP.
| 10607567510996 - TigerPaw Pro Left Atrial Appendage Closure Device | 2025-01-31 Left Atrial Appendage Closure Device 35mm - 7 pin |
| 10607567107301 - MEGA 8Fr. 50cc IAB | 2024-12-09 MEGA 8Fr. 50cc IAB |
| 10607567107318 - MEGA 8Fr. 50cc IAB (with APA) | 2024-12-09 MEGA 8Fr. 50cc IAB (with APA) |
| 10607567107479 - Arrow Pump Adapter - 5 Pack - for use with the Mega 8 Fr. 50cc IAB only | 2024-12-09 Arrow Pump Adapter - 5 Pack - for use with the Mega 8 Fr. 50cc IAB only |
| 10607567107516 - Catheter Extenders for 50cc IAB’s | 2024-12-09 Catheter Extenders for 50cc IAB’s |
| 10607567108001 - MEGA 8Fr. 50cc IAB (with Statlock) | 2024-12-09 MEGA 8Fr. 50cc IAB (with Statlock) |
| 10607567108018 - MEGA 8Fr. 50cc IAB (with Statlock & APA) | 2024-12-09 MEGA 8Fr. 50cc IAB (with Statlock & APA) |
| 10607567108063 - SENSATION PLUS 7.5Fr. 40cc IAB & Accessories | 2024-12-09 SENSATION PLUS 7.5Fr. 40cc IAB & Accessories |
Trademark Results [Raceway]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.