T-Cup® Compact ACDOA-6125
GUDID 00817504020560
T-Cup® Compact Multi-Drug Urine Test Cup
Instant Technologies, Inc.
Multiple drugs of abuse IVD, kit, immunochromatographic test (ICT), rapid| Primary Device ID | 00817504020560 |
| NIH Device Record Key | aa292052-cf0c-43d6-bb88-a8bf5ae462a3 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | T-Cup® Compact |
| Version Model Number | ACDOA-6125 |
| Catalog Number | ACDOA-6125 |
| Company DUNS | 019410187 |
| Company Name | Instant Technologies, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-800-340-4029 |
| qa.portsmouth@alere.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 4 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00817504020560 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K133968
FDA Product Code
| LDJ | Enzyme Immunoassay, Cannabinoids |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-05-01 |
| Device Publish Date | 2019-04-23 |
On-Brand Devices [T-Cup® Compact ]
| 00817504020560 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 00817405021048 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 00817405021031 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 00817405021024 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 00817405021017 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 00817405021000 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 00817405020560 | T-Cup® Compact Multi-Drug Urine Test Cup |
| 10817405021298 | UScreen Squared Integrated CLIA Waived Cup with Adulterants (CR/NI/pH/OX/SG) 14 Panel Drug Scree |
Trademark Results [T-Cup]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
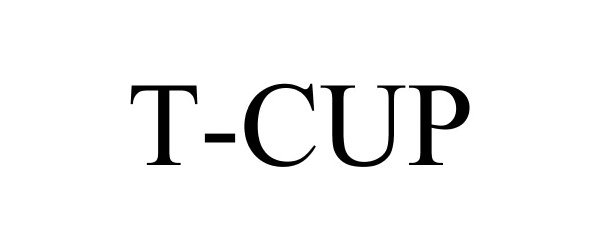 T-CUP 87151774 5212506 Live/Registered |
Wondfo USA Co., Ltd. 2016-08-26 |
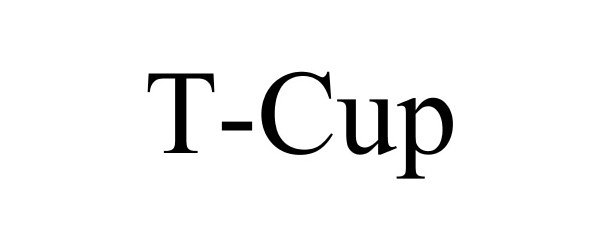 T-CUP 86362305 not registered Dead/Abandoned |
Minnesota Medtech 2014-08-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.