Pagoda
GUDID 00822409022991
Diameter 8.5 mm x 55 mm Polyaxial Screw
Ortho Development Corporation
Bone-screw internal spinal fixation system, sterile| Primary Device ID | 00822409022991 |
| NIH Device Record Key | 976c16fd-bbf5-4fa3-9c19-f96711f136cb |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Pagoda |
| Version Model Number | 752-8555-11A |
| Company DUNS | 876542390 |
| Company Name | Ortho Development Corporation |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com | |
| Phone | +1(801)553-9991 |
| customerservice@odev.com |
Device Dimensions
| Length | 55 Millimeter |
| Length | 55 Millimeter |
| Length | 55 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
| Outer Diameter | 8.5 Millimeter |
| Length | 55 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00822409022991 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K131785
FDA Product Code
| MNI | ORTHOSIS, SPINAL PEDICLE FIXATION |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-07-15 |
On-Brand Devices [Pagoda]
| 00822409034222 | Diameter 5.5 mm x 500 mm Straight Spinal Rod |
| 00822409034215 | Diameter 5.5 mm x 405 mm Straight Spinal Rod |
| 00822409034208 | Diameter 5.5 mm x 200 mm Straight Spinal Rod |
| 00822409034192 | Diameter 5.5 mm x 105 mm Straight Spinal Rod |
| 00822409034185 | Diameter 5.5 mm x 90 mm Straight Spinal Rod |
| 00822409034178 | Diameter 5.5 mm x 85 mm Straight Spinal Rod |
| 00822409034161 | Diameter 5.5 mm x 80 mm Straight Spinal Rod |
| 00822409034154 | Diameter 5.5 mm x 75 mm Straight Spinal Rod |
| 00822409034147 | Diameter 5.5 mm x 70 mm Straight Spinal Rod |
| 00822409034130 | Diameter 5.5 mm x 65 mm Straight Spinal Rod |
| 00822409034123 | Diameter 5.5 mm x 60 mm Straight Spinal Rod |
| 00822409034116 | Diameter 5.5 mm x 55 mm Straight Spinal Rod |
| 00822409034109 | Diameter 5.5 mm x 50 mm Straight Spinal Rod |
| 00822409034093 | Diameter 5.5 mm x 45 mm Straight Spinal Rod |
| 00822409034086 | Diameter 5.5 mm x 40 mm Straight Spinal Rod |
| 00822409034079 | Diameter 5.5 mm x 35 mm Straight Spinal Rod |
| 00822409034062 | Diameter 5.5 mm x 30 mm Straight Spinal Rod |
| 00822409034055 | Diameter 5.5 mm x 150 mm Curved Rod Ti, |
| 00822409034048 | Diameter 5.5 mm x 135 mm Curved Rod Ti |
| 00822409034031 | Diameter 5.5 mm x 120 mmCurved Rod Ti |
| 00822409034024 | Diameter 5.5 mm x 105 mm Curved Spinal Rod |
| 00822409034017 | Diameter 5.5 mm x 90 mm Curved Spinal Rod |
| 00822409034000 | Diameter 5.5 mm x 85 mm Curved Spinal Rod |
| 00822409033997 | Diameter 5.5 mm x 80 mm Curved Spinal Rod |
| 00822409033980 | Diameter 5.5 mm x 75 mm Curved Spinal Rod |
| 00822409033973 | Diameter 5.5 mm x 70 mm Curved Spinal Rod |
| 00822409033966 | Diameter 5.5 mm x 65 mm Curved Spinal Rod |
| 00822409033959 | Diameter 5.5 mm x 60 mm Curved Spinal Rod |
| 00822409033942 | Diameter 5.5 mm x 55 mm Curved Spinal Rod |
| 00822409033935 | Diameter 5.5 mm x 50 mm Curved Spinal Rod |
| 00822409033928 | Diameter 5.5 mm x 45 mm Curved Spinal Rod |
| 00822409033911 | Diameter 5.5 mm x 40 mm Curved Spinal Rod |
| 00822409033904 | Diameter 5.5 mm x 35 mm Curved Spinal Rod |
| 00822409033898 | Diameter 5.5 mm x 30 mm Curved Spinal Rod |
| 00822409029938 | Diameter 8.5 mm x 90 mm Monoaxial Screw |
| 00822409029921 | Diameter 8.5 mm x 80 mm Monoaxial Screw |
| 00822409029914 | Diameter 8.5 mm x 70 mm Monoaxial Screw |
| 00822409029891 | Diameter 8.5 mm x 60 mm Monoaxial Screw |
| 00822409029860 | Diameter 8.5 mm x 60 mm Polyaxial Reduction Screw |
| 00822409029853 | Diameter 8.5 mm x 60 mm Polyaxial Screw |
| 00822409029839 | Diameter 8.5 mm x 55 mm Monoaxial Screw |
| 00822409029808 | Diameter 8.5 mm x 55 mm Polyaxial Reduction Screw |
| 00822409029792 | Diameter 8.5 mm x 55 mm Polyaxial Screw |
| 00822409029778 | Diameter 8.5 mm x 50 mm Monoaxial Screw |
| 00822409029747 | Diameter 8.5 mm x 50 mm Polyaxial Reduction Screw |
| 00822409029730 | Diameter 8.5 mm x 50 mm Polyaxial Screw |
| 00822409029716 | Diameter 8.5 mm x 45 mm Monoaxial Screw |
| 00822409029686 | Diameter 8.5 mm x 45 mm Polyaxial Reduction Screw |
| 00822409029679 | Diameter 8.5 mm x 45 mm Polyaxial Screw |
| 00822409029655 | Diameter 8.5 mm x 40 mm Monoaxial Screw |
Trademark Results [Pagoda]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
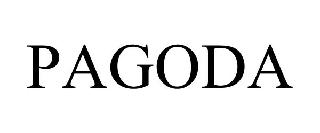 PAGODA 98554941 not registered Live/Pending |
TXDC, L.P. 2024-05-16 |
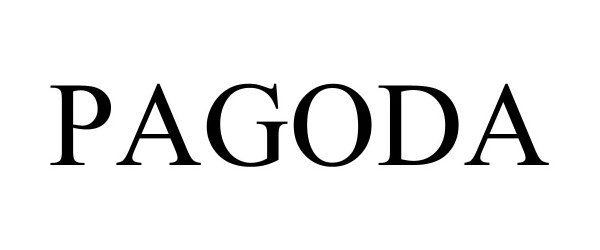 PAGODA 97854431 not registered Live/Pending |
RYCROFT HOLDINGS LLC 2023-03-23 |
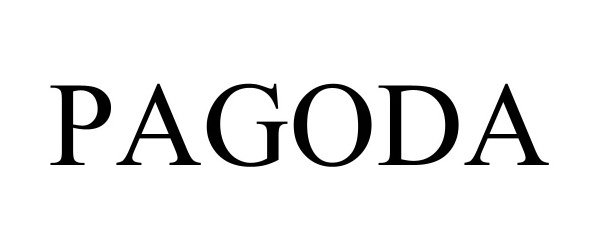 PAGODA 97462358 not registered Live/Pending |
NEAR Stiftung 2022-06-16 |
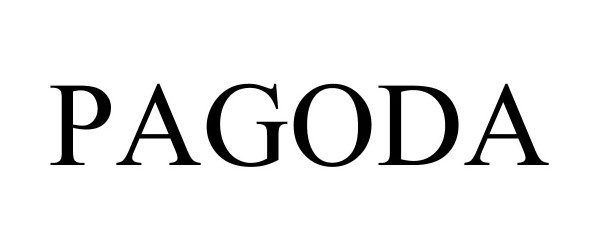 PAGODA 90634325 not registered Live/Pending |
Schwan's IP, LLC 2021-04-09 |
 PAGODA 90151193 not registered Live/Pending |
Drake Holdings, Inc. 2020-09-01 |
 PAGODA 88732363 not registered Live/Pending |
Labcon, North America 2019-12-18 |
 PAGODA 88523515 not registered Live/Pending |
Velvet Pagoda Limited 2019-07-19 |
 PAGODA 87583778 not registered Dead/Abandoned |
Schwan's IP, LLC 2017-08-25 |
 PAGODA 87544682 5416791 Live/Registered |
XIAMEN WEIJIA SHOES CO., LTD. 2017-07-27 |
 PAGODA 87231287 5342360 Live/Registered |
TXDC, L.P. 2016-11-09 |
 PAGODA 86454239 4899371 Live/Registered |
Schwan's IP, LLC 2014-11-14 |
 PAGODA 86282021 4896177 Live/Registered |
Schwan's IP, LLC 2014-05-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.