Kompressor 2804014A
GUDID 00885556868942
REAR FOOT RECONSTRUCTION PLATE, 66MM, 14 HOLES
Smith & Nephew, Inc.
Orthopaedic prosthesis implantation positioning instrument, reusable| Primary Device ID | 00885556868942 |
| NIH Device Record Key | bf2dcadc-bbfb-4616-a84d-3955131b1310 |
| Commercial Distribution Status | In Commercial Distribution |
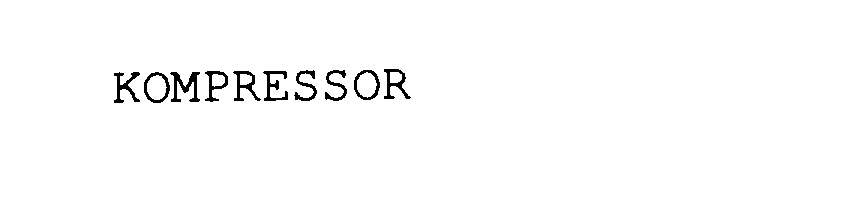
| Brand Name | Kompressor |
| Version Model Number | 2804014 |
| Catalog Number | 2804014A |
| Company DUNS | 109903521 |
| Company Name | Smith & Nephew, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
| Special Storage Condition, Specify | Between 0 and 0 *: - |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00885556868942 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K100502
FDA Product Code
| HRS | Plate, fixation, bone |
| LXH | Orthopedic manual surgical instrument |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
[00885556868942]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-26 |
| Device Publish Date | 2023-06-18 |
On-Brand Devices [Kompressor]
| 00885556868966 | DEPTH GAGE, FIXED ANGLE SURFIX, DIAMETER 3.5 MM |
| 00885556868942 | REAR FOOT RECONSTRUCTION PLATE, 66MM, 14 HOLES |
| 00885556869062 | MINI K-WIRE 0.9MM |
Trademark Results [Kompressor]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.