Single-Site
GUDID 00886874113615
CURVED SCISSORS
INTUITIVE SURGICAL, INC.
Robotic surgical scissors| Primary Device ID | 00886874113615 |
| NIH Device Record Key | c936fdae-5832-4169-8ffd-618d589db515 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Single-Site |
| Version Model Number | 478057 |
| Company DUNS | 938647021 |
| Company Name | INTUITIVE SURGICAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00886874113615 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K152448
FDA Product Code
| GCJ | Laparoscope, general & plastic surgery |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
[00886874113615]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 13 |
| Public Version Date | 2020-03-27 |
| Device Publish Date | 2016-07-08 |
On-Brand Devices [Single-Site]
| 00886874117293 | SUCTION IRRIGATOR |
| 00886874113790 | CANNULA, GROUNDED, CAM |
| 00886874113752 | WRISTED NEEDLE DRIVER |
| 10886874113735 | FENESTRATED BIPOLAR FORCEPS |
| 00886874113721 | PERMANENT CAUTERY HOOK |
| 00886874113714 | CURVED NEEDLE DRIVER |
| 10886874113704 | BIPOLAR MARYLAND |
| 00886874113691 | CANNULA, CURVED, CAM LEFT |
| 00886874113684 | CANNULA, CURVED, CAM RIGHT |
| 10886874113674 | ENDOSCOPE PORT |
| 00886874113660 | CANNULA, CURVED, CAM LEFT |
| 00886874113653 | CANNULA, CURVED, CAM RIGHT |
| 00886874113646 | CANNULA |
| 00886874113639 | CROCKODILE GRASPER |
| 00886874113622 | FUNDUS GRASPER |
| 00886874113615 | CURVED SCISSORS |
| 00886874113608 | CADIERE FORCEPS |
| 00886874113592 | SUCTION IRRIGATOR |
| 00886874113585 | CLIP APPLIER, MEDIUM-LARGE |
| 00886874113561 | MARYLAND DISSECTOR |
| 00886874113554 | ACCESSORY OBTRUATOR |
| 00886874113547 | BLUNT OBTRUATOR |
| 30886874112725 | CANNULA SEAL |
| 00886874112076 | BLUNT LATCHING OBTURATOR |
| 00886874112052 | ACCESSORY CANNULA |
| 00886874112045 | FLEXIBLE BLUNT OBTRUATOR |
| 00886874112038 | CURVED CANNULA ARM2 |
| 00886874112021 | CURVED CANNULA ARM1 |
| 30886874112015 | ENDOSCOPE PORT |
| 00886874112007 | FLEXIBLE BLUNT OBTRUATOR |
| 00886874111994 | CURVED CANNULA ARM2 |
| 00886874111987 | CURVED CANNULA ARM1 |
| 00886874111970 | ACCESSORY CANNULA |
Trademark Results [Single-Site]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
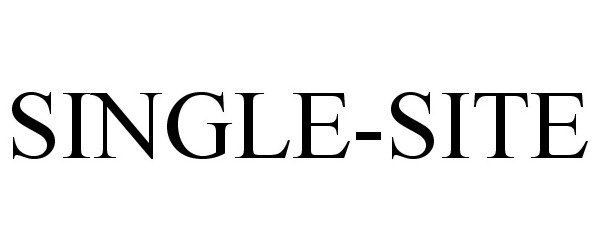 SINGLE-SITE 85652245 4370202 Live/Registered |
INTUITIVE SURGICAL OPERATIONS, INC. 2012-06-14 |
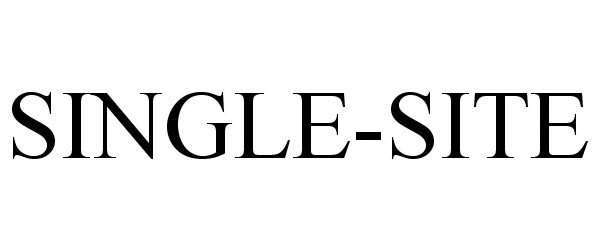 SINGLE-SITE 77882345 not registered Dead/Abandoned |
Intuitive Surgical, Inc. 2009-11-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.