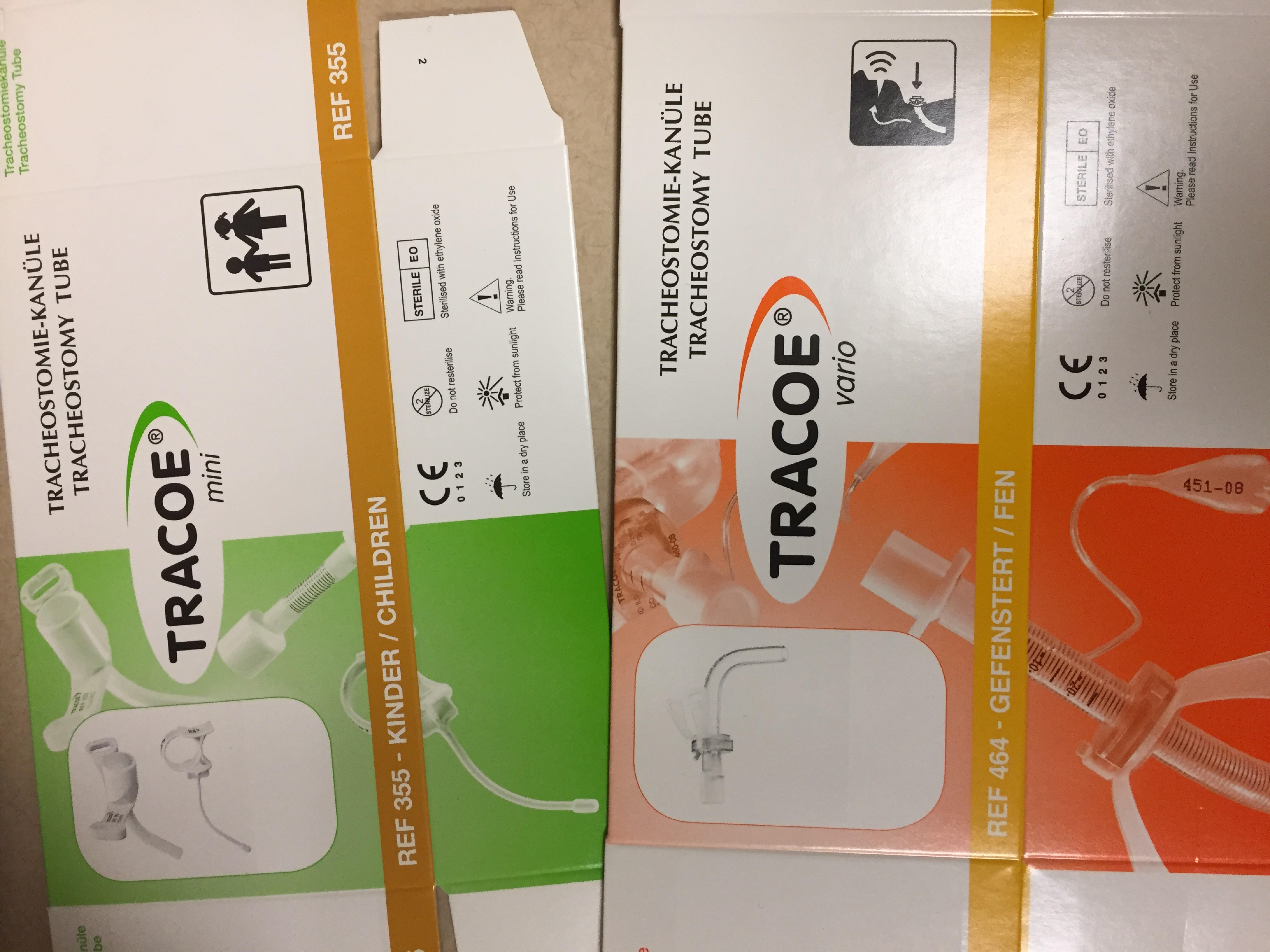
TRACOE® modular
GUDID 04035324057016
modular Stopper for temporary closure of the 22 mm housing, 5 units
Tracoe medical GmbH
Tracheostomy tube decannulation cap| Primary Device ID | 04035324057016 |
| NIH Device Record Key | 98461997-3387-4aaf-bf9e-41261050112e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TRACOE® modular |
| Version Model Number | REF 621 |
| Company DUNS | 319414785 |
| Company Name | Tracoe medical GmbH |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com | |
| Phone | +49613691690 |
| info@tracoe.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Special Storage Condition, Specify | Between 0 and 0 *no Special Storage Conditions |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
| Storage Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from rain. Keep away from sunlight. Do not use if package is damaged and consult IFU. Do not resterilize. |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04035324057016 [Primary] |
| GS1 | 05701780328371 [Unit of Use] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K961449
FDA Product Code
| JOH | Tube tracheostomy and tube cuff |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-26 |
| Device Publish Date | 2023-06-17 |
On-Brand Devices [TRACOE® modular]
| 04035324057016 | modular Stopper for temporary closure of the 22 mm housing, 5 units |
| 04035324054718 | TRACOE humid assist I, 50 units |
| 04035324054510 | TRACOE phon assist I |
| 04035324019489 | TRACOE humid assist mini, 50 units |
| 04035324012923 | TRACOE phon assist I with Oxygen Supply Port |
| 04035324011704 | TRACOE humid assist III, 50 units |
Trademark Results [TRACOE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRACOE 75072311 2117376 Live/Registered |
TRACOE MEDICAL GMBH 1996-03-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.