Suprasorb 162461
GUDID 04068225066962
Lohmann & Rauscher International GmbH & Co KG
Synthetic polymer semi-permeable film dressing, adhesive| Primary Device ID | 04068225066962 |
| NIH Device Record Key | a3e8ce16-1adb-4aca-a53f-dd54dcb4fb61 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Suprasorb |
| Version Model Number | F Protect 5 x 7 cm |
| Catalog Number | 162461 |
| Company DUNS | 315505235 |
| Company Name | Lohmann & Rauscher International GmbH & Co KG |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *keep dry |
| Special Storage Condition, Specify | Between 0 and 0 *keep away from sunlight |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04068225066955 [Primary] |
| GS1 | 04068225066962 [Package] Contains: 04068225066955 Package: SC [100 Units] In Commercial Distribution |
| GS1 | 04068225066979 [Package] Package: TC [10 Units] In Commercial Distribution |
FDA Product Code
| NAD | Dressing, Wound, Occlusive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-11-25 |
| Device Publish Date | 2024-11-15 |
On-Brand Devices [Suprasorb]
| 04021447549552 | A Wound Dressing 10 x 12,5 cm |
| 04021447031002 | P sacrum self-adhesive |
| 04021447204666 | F 20 x 30 cm |
| 04021447204659 | F 10 cm x 20 cm |
| 04021447204642 | F 10 cm x 25 cm |
| 04021447204635 | F 10 cm x 12 cm |
| 04021447204628 | F 10 cm x 12 cm |
| 04021447204611 | F 5 x 7 cm |
| 04021447204604 | F 5 x 7 cm |
| 04021447204451 | A Rope 30cm /2g |
| 04021447204420 | A Wound Dressing 10 x 20 cm |
| 04021447204413 | A Wound Dressing 10 x 10 cm |
| 04021447204406 | A Wound Dressing 5 x 5 cm |
| 04021447204192 | P self-adhesive |
| 04021447204185 | P self-adhesive |
| 04021447204178 | P self-adhesive |
| 04021447204161 | P self-adhesive |
| 04021447204093 | P non adhesive |
| 04021447204086 | P non adhesive |
| 04021447204079 | P non adhesive |
| 04021447204062 | P non adhesive |
| 04021447204055 | P non adhesive |
| 04068225067112 | F Protect 20 x 30 cm |
| 04068225067082 | F Protect 15 cm x 20 cm |
| 04068225067051 | F Protect 10 cm x 25 cm |
| 04068225067020 | F Protect 10 cm x 12 cm |
| 04068225066993 | F Protect 10 cm x 12 cm |
| 04068225066962 | F Protect 5 x 7 cm |
| 04068225066931 | F Protect 5 x 7 cm |
Trademark Results [Suprasorb]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
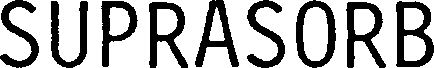 SUPRASORB 79315321 not registered Live/Pending |
Lohmann & Rauscher International GmbH &Co. KG 2021-06-14 |
 SUPRASORB 76317307 2789369 Live/Registered |
LOHMANN & RAUSCHER, INC. 2001-09-26 |
 SUPRASORB 75704480 not registered Dead/Abandoned |
RAUSCHER & Co. Verbandstoff- und Wattefabriken Ges.mbH 1999-05-12 |
 SUPRASORB 75006582 2107710 Dead/Cancelled |
Medipro Internacional, S.A. De C.V. 1995-10-16 |
 SUPRASORB 74305604 not registered Dead/Abandoned |
Medipro Internacional, S.A. De C.V. 1992-08-19 |
 SUPRASORB 73438954 not registered Dead/Abandoned |
Standard Terry Mills, Inc. 1983-08-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.