MULTI-GUIDE 60-15420
GUDID 04546540246905
DRILL, WL 20MM, DENTAL-SHAFT
Stryker Leibinger GmbH & Co. KG
Fluted surgical drill bit, single-use, non-sterile| Primary Device ID | 04546540246905 |
| NIH Device Record Key | 7b49797b-d25c-49a7-b679-ec8e830f6261 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MULTI-GUIDE |
| Version Model Number | 60-15420 |
| Catalog Number | 60-15420 |
| Company DUNS | 316153956 |
| Company Name | Stryker Leibinger GmbH & Co. KG |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
| Length | 20 Millimeter |
| Length | 135 Millimeter |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04546540246905 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K070876
FDA Product Code
| HBE | DRILLS, BURRS, TREPHINES & ACCESSORIES (SIMPLE, POWERED) |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
[04546540246905]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-11-22 |
| Device Publish Date | 2016-08-24 |
On-Brand Devices [MULTI-GUIDE]
| 04546540257796 | TROCAR F. DRILL GUIDE |
| 04546540257789 | CHEEK RETRACTOR F. DRILL GUIDE |
| 04546540257772 | SINGLE DRILL GUIDE INSERT |
| 04546540257765 | DOUBLE DRILL GUIDE INSERT |
| 04546540257758 | HANDLE FOR DRILL GUIDE |
| 04546540257734 | TRANSVERSE ACTIVATION TOOL |
| 04546540257727 | ANGULAR ACTIVATION TOOL |
| 04546540257710 | LINEAR ACTIVATION TOOL, COMBINATION |
| 04546540257697 | PIN CLAMP, RIGHT |
| 04546540257680 | MANDIBULAR DISTRACTION DEVICE |
| 04546540257673 | MANDIBULAR DISTRACTION DEVICE |
| 04546540246905 | DRILL, WL 20MM, DENTAL-SHAFT |
| 04546540210289 | STORAGE CONTAINER |
| 04546540133946 | BLADE FOR THREAD. PINS AND CLAMP. SCREWS |
| 34546540259074 | SELF-DRILLING PINS, AUTO PILOT |
| 34546540259067 | SELF-DRILLING PINS, AUTO PILOT |
| 34546540259050 | SELF-DRILLING PINS, AUTO PILOT |
Trademark Results [MULTI-GUIDE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
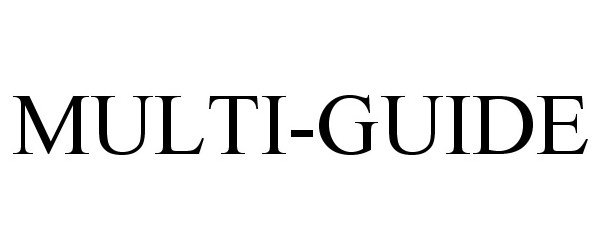 MULTI-GUIDE 85251908 4163210 Live/Registered |
STRYKER CORPORATION 2011-02-25 |
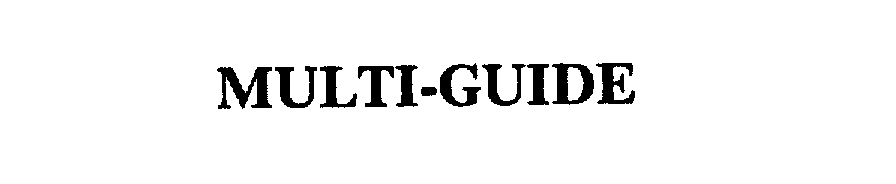 MULTI-GUIDE 75497345 2267056 Live/Registered |
STRYKER LEIBINGER GMBH & CO. KG 1998-06-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.