BOON
GUDID 06938327900458
2-200 mL Syringes 2-Fill Straws 1-72"" Y Coiled Tubing with Dual Check Valves"
Shenzhen Boon Medical Supply Co., Ltd.
Angiographic syringe| Primary Device ID | 06938327900458 |
| NIH Device Record Key | c3dbdc85-19f1-4564-b269-f546aa37cd7b |
| Commercial Distribution Discontinuation | 2020-04-26 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | BOON |
| Version Model Number | 100113-A |
| Company DUNS | 529174252 |
| Company Name | Shenzhen Boon Medical Supply Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06938327900458 [Primary] |
| GS1 | 26938327900452 [Package] Package: Carton [20 Units] Discontinued: 2020-04-26 Not in Commercial Distribution |
| GS1 | 26938327900458 [Package] Package: Carton [20 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K151960
FDA Product Code
| DXT | Injector And Syringe, Angiographic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 7 |
| Public Version Date | 2020-04-27 |
| Device Publish Date | 2017-05-26 |
On-Brand Devices [BOON]
| 06938327900618 | 1-100ml Syringe 1-Large Spike |
| 06938327900595 | 1-200 mL Syringe 1-Large Spike 1-72” Coiled Tubing |
| 06938327900465 | 2-200 mL Syringes 2-Large Spikes 1-72"" |
| 06938327900458 | 2-200 mL Syringes 2-Fill Straws 1-72" |
| 06938327900236 | 1200mm 1200PSI High Pressure Braided Line with Rotating Luer |
| 06938327900229 | 1000mm 1200PSI High Pressure Braided Line with Rotating Luer |
| 06938327900212 | 750mm High Pressure Braided Line with Rotating Luer |
| 06938327900205 | 500mm 1200PSI High Pressure Braided Line with Rotating Luer |
| 06938327900069 | 1-130ml Syringe 1-Fill Straw |
| 06938327900021 | 2-200 mL Syringes 2-Fill Straws & 2 Large Spikes 1-72"" Y Coiled Tubing with Dual Check |
| 06938327900014 | 1-200 mL Syringe 1-Fill Straw 1-72” Coiled Tubing |
Trademark Results [BOON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
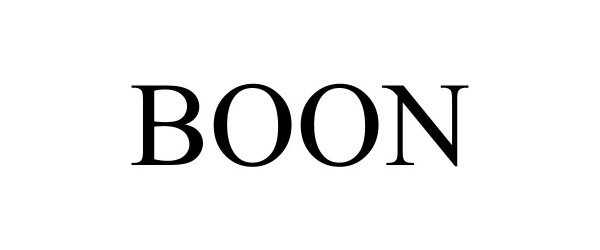 BOON 98608791 not registered Live/Pending |
AMINOMEGA LLC 2024-06-19 |
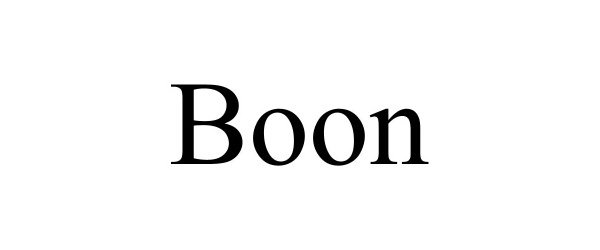 BOON 98422451 not registered Live/Pending |
PIXO Inc. 2024-02-27 |
 BOON 97592230 not registered Live/Pending |
Marr, Scott 2022-09-15 |
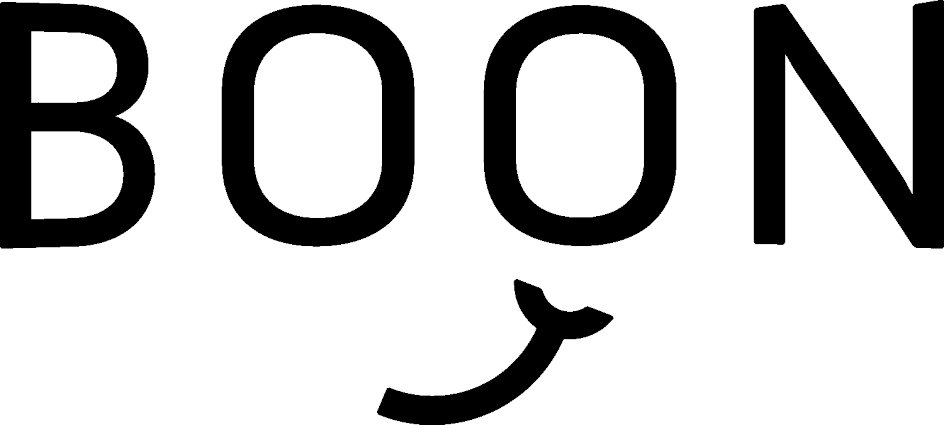 BOON 97111114 not registered Live/Pending |
Simmons, Alex 2021-11-05 |
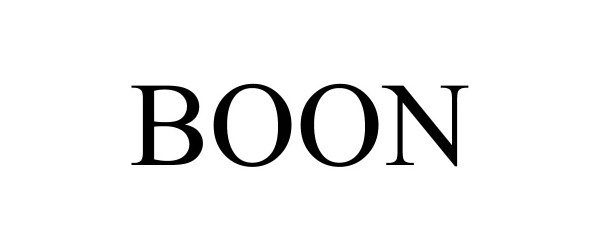 BOON 97091687 not registered Live/Pending |
FishFins LLC 2021-10-25 |
 BOON 90903304 not registered Live/Pending |
GetMyDNA LLC 2021-08-26 |
 BOON 90430602 not registered Live/Pending |
Synergy Products, Inc. 2020-12-30 |
 BOON 90199571 not registered Live/Pending |
Vkali of Texas, Inc. 2020-09-22 |
 BOON 90191550 not registered Live/Pending |
Advanced Food Concepts, Incorporated 2020-09-18 |
 BOON 90190472 not registered Live/Pending |
Boon Nutrition LLC 2020-09-18 |
 BOON 88744193 not registered Live/Pending |
The Boon Group LLC 2020-01-01 |
 BOON 88700132 not registered Live/Pending |
Simmons, Alex 2019-11-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.