Tool Kit
GUDID 06938695520012
KMC Kyphoplasty System consists of four components: Balloon Catheter Tool Kit Puncture Needle KMC Medical Balloon Catheter Syringe Pump
Shanghai Kinetic Medical Co., Ltd.
Bone access channel kit| Primary Device ID | 06938695520012 |
| NIH Device Record Key | c0e18e91-d508-478d-8188-9c148b9eed52 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Tool Kit |
| Version Model Number | FG2001 |
| Company DUNS | 528198569 |
| Company Name | Shanghai Kinetic Medical Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Operating and Storage Conditions
| Handling Environment Temperature | Between 18 Degrees Celsius and 25 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06938695520012 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K113742
FDA Product Code
| NDN | Cement, Bone, Vertebroplasty |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-01-14 |
| Device Publish Date | 2020-01-06 |
On-Brand Devices [Tool Kit]
| 06938695520029 | KMC Kyphoplasty System consists of four components: Balloon Catheter Tool Kit Puncture Needle KM |
| 06938695520012 | KMC Kyphoplasty System consists of four components: Balloon Catheter Tool Kit Puncture Needle KM |
Trademark Results [Tool Kit]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
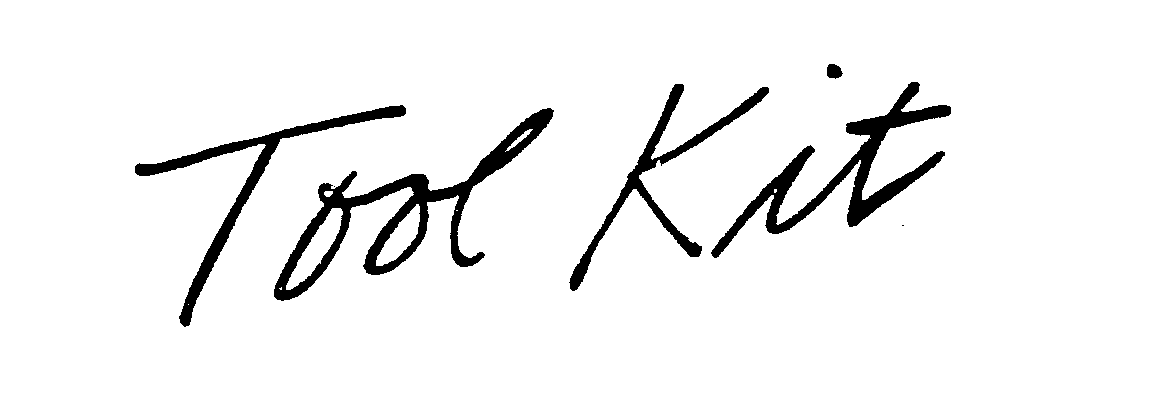 TOOL KIT 78288180 3187871 Live/Registered |
Grayson O. Company 2003-08-15 |
 TOOL KIT 76371954 not registered Dead/Abandoned |
Unger, Gerhard 2002-02-15 |
 TOOL KIT 74252744 1733006 Dead/Cancelled |
Tool Kit Industries, Inc. 1992-03-06 |
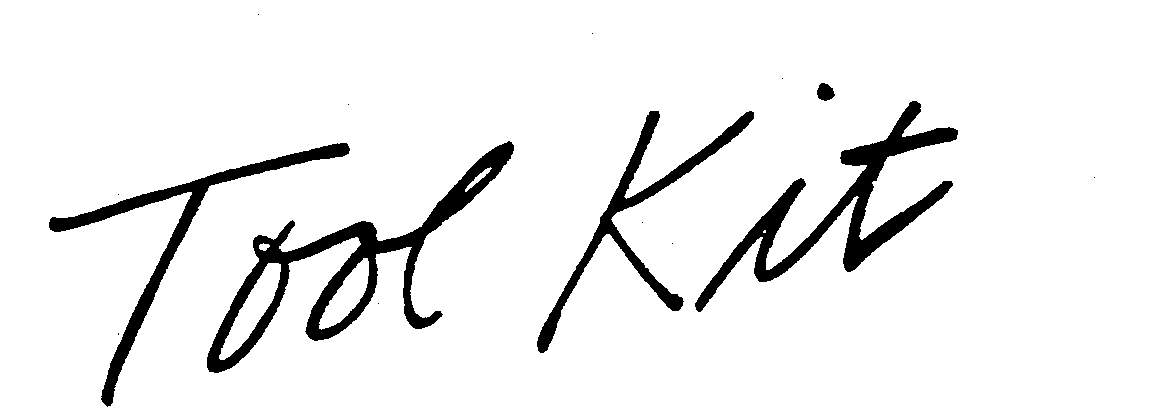 TOOL KIT 73578116 1407291 Live/Registered |
GRAYSON O COMPANY 1986-01-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.