TEC.BEAN
GUDID 06953825903091
Shenzhen Urion Technology Co., Ltd
Automatic-inflation electronic sphygmomanometer, portable, arm/wrist| Primary Device ID | 06953825903091 |
| NIH Device Record Key | 298daf5b-6d7e-47c2-81c3-55af0151c7d2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TEC.BEAN |
| Version Model Number | U80Q |
| Company DUNS | 421294358 |
| Company Name | Shenzhen Urion Technology Co., Ltd |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06953825903091 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K160019
FDA Product Code
| DXN | System, Measurement, Blood-Pressure, Non-Invasive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 6 |
| Public Version Date | 2020-04-09 |
| Device Publish Date | 2017-09-21 |
Devices Manufactured by Shenzhen Urion Technology Co., Ltd
| 16953825910829 - SAKER | 2026-02-02 |
| 16953825910768 - AlphagoMed | 2026-01-14 |
| 16953825910607 - FOTOSY | 2025-11-25 |
| 16953825910614 - FOTOSY | 2025-11-25 |
| 16953825910546 - Tovendor | 2025-11-19 |
| 16953825901070 - AlphaMed | 2025-09-08 |
| 16953825910041 - Alphamed | 2025-08-08 |
| 16953825909960 - N/A | 2025-07-10 |
Trademark Results [TEC.BEAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
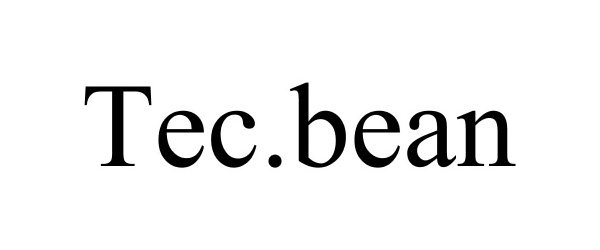 TEC.BEAN 87646343 5438352 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-10-16 |
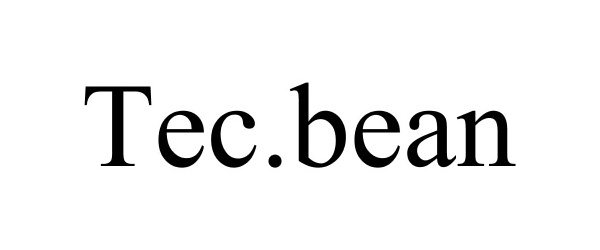 TEC.BEAN 87622126 5438306 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-09-26 |
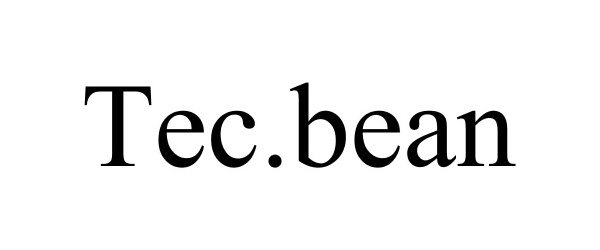 TEC.BEAN 87613455 5553367 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-09-19 |
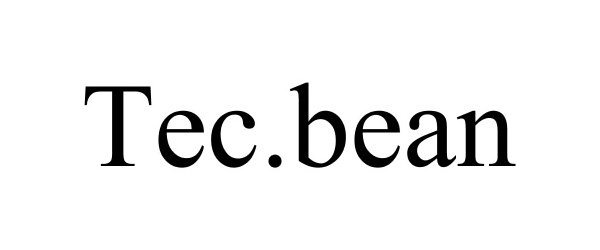 TEC.BEAN 87606050 5438268 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd. 2017-09-13 |
 TEC.BEAN 87595118 5437671 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd. 2017-09-04 |
 TEC.BEAN 87589065 5386762 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd. 2017-08-29 |
 TEC.BEAN 87587264 5433108 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-08-29 |
 TEC.BEAN 87585530 5432985 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-08-28 |
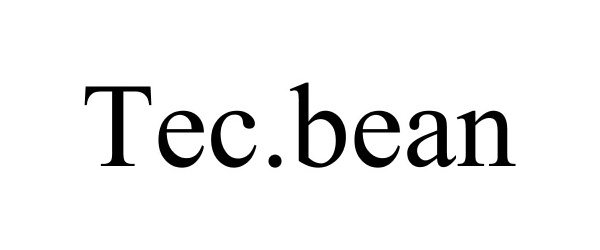 TEC.BEAN 87585491 5553295 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-08-28 |
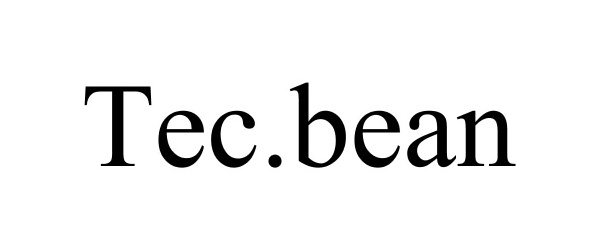 TEC.BEAN 87584911 5418263 Live/Registered |
Shenzhen Valuelink E-Commerce Co.,Ltd 2017-08-26 |
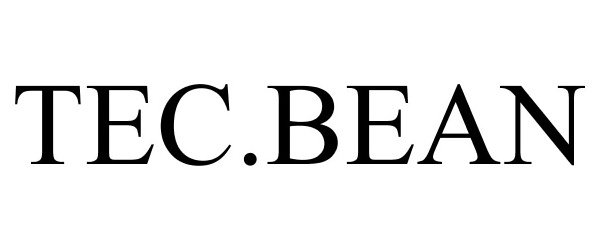 TEC.BEAN 86294327 4667112 Live/Registered |
SHENZHEN VALUELINK E-COMMERCE CO.,LTD. 2014-05-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.