Revel
GUDID 10190752157071
REVEL Ventilator
VYAIRE MEDICAL, INC.
Neonatal/adult intensive-care ventilator| Primary Device ID | 10190752157071 |
| NIH Device Record Key | 2c508df1-bbae-431e-a71c-3cb96d4a33c7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Revel |
| Version Model Number | 19260-001 |
| Company DUNS | 080456871 |
| Company Name | VYAIRE MEDICAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10190752157071 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K103211
FDA Product Code
| CBK | Ventilator, Continuous, Facility Use |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-03-28 |
| Device Publish Date | 2024-03-20 |
Devices Manufactured by VYAIRE MEDICAL, INC.
| 10190752190535 - LTV | 2025-09-03 LTV FLOOR STAND |
| 10190752191518 - bellavista | 2025-08-05 bellaivsta Nurse Call Cable Normally Open |
| 10190752188860 - bellavista | 2025-08-04 bellaivsta Nurse Call Connector |
| 10190752191525 - bellavista | 2025-08-04 bellaivsta Nurse Call Connector |
| 10190752130364 - LTV2 Ventilator | 2025-07-24 TABLE STAND, LTV/LTV2 |
| 20190752201061 - 3100A HFOV | 2025-07-24 FILTER PK10 3100A |
| 10190752185197 - LTV2 | 2025-07-24 O2 CYLINDER MOUNT, LTV2 FLOOR STAND |
| 10190752188754 - LTV2 Ventilator | 2025-07-24 Nurse Call Cable, Normally Open |
Trademark Results [Revel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REVEL 98618294 not registered Live/Pending |
Brooks Sports, Inc. 2024-06-25 |
 REVEL 98483684 not registered Live/Pending |
Humble Snacks Inc. 2024-04-04 |
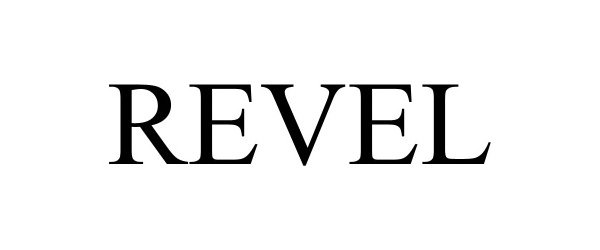 REVEL 98195336 not registered Live/Pending |
AGS LLC 2023-09-25 |
 REVEL 98168450 not registered Live/Pending |
Globus Medical, Inc. 2023-09-07 |
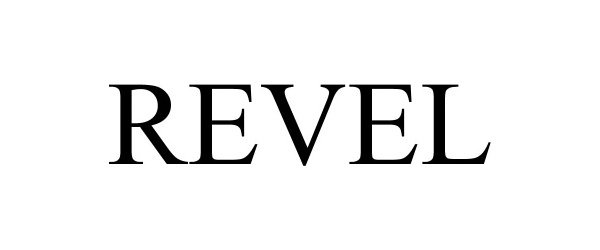 REVEL 97975691 not registered Live/Pending |
REVEL MOMENTS INC. 2021-09-10 |
 REVEL 97897522 not registered Live/Pending |
Revel Brand Group LLC 2023-04-19 |
 REVEL 97894115 not registered Live/Pending |
P.T. Djarum 2023-04-18 |
 REVEL 97870075 not registered Live/Pending |
Virox Technologies Inc. 2023-04-03 |
 REVEL 97840413 not registered Live/Pending |
Revel Coach, LLC 2023-03-15 |
 REVEL 97758038 not registered Live/Pending |
Nano Hearing Tech Opco, LLC 2023-01-17 |
 REVEL 97679876 not registered Live/Pending |
Sterling Perfumes Industries LLC 2022-11-16 |
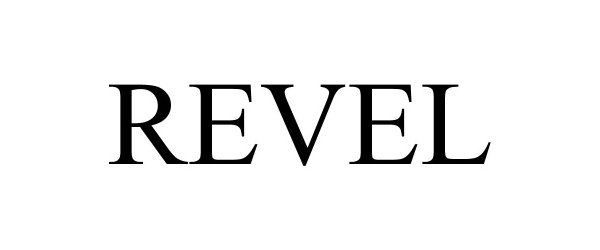 REVEL 97330389 not registered Live/Pending |
Queen Creek Veterinary Group, PLLC 2022-03-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.