VALULINE 5251-532
GUDID 10366975012989
MASK VALULINE LEVEL 3 BLU BX50
BENCO DENTAL SUPPLY CO.
Surgical/medical face mask, single-use| Primary Device ID | 10366975012989 |
| NIH Device Record Key | 0f9c8f98-31a9-4a02-80fb-692a5f3c02a8 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | VALULINE |
| Version Model Number | 5251-532 |
| Catalog Number | 5251-532 |
| Company DUNS | 015108087 |
| Company Name | BENCO DENTAL SUPPLY CO. |
| Device Count | 50 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a cool dry place. Avoid excessive heat (40°C or 104°F) |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00366975012982 [Primary] |
| GS1 | 00366975013125 [Unit of Use] |
| GS1 | 10366975012989 [Package] Contains: 00366975012982 Package: CASE [10 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K051291
FDA Product Code
| FXX | Mask, Surgical |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2020-05-06 |
| Device Publish Date | 2018-01-31 |
On-Brand Devices [VALULINE]
| 10366975012989 | MASK VALULINE LEVEL 3 BLU BX50 |
| 10366975012972 | MASK VALULINE LEVEL 3 PNK BX50 |
| 00366975010056 | VALULINE VPS MONO FS REFILL |
| 00366975010070 | VALULINE VPS MONO FS INTRO KT |
| 00366975010049 | VALULINE VPS HB FS REFILL |
| 00366975010063 | VALULINE VPS HB FS INTRO KT |
| 10366975003031 | POUCH VALULINE 2.25X4 BX200 |
| 10366975003024 | POUCH VALULINE 2.75X9 BX200 |
| 10366975003017 | POUCH VALULINE 5.25X10 BX200 |
| 10366975003000 | POUCH VALULINE 3.5X9 BX500 |
| 00366975002990 | POUCH VALULINE 3.5X9 BX200 |
| 10366975002980 | POUCH VALULINE 3.5X5.25 BX200 |
| 10366975035889 | 5519-271 |
| 00366975060266 | 5749-326 |
| 00366975060259 | 5749-317 |
| 00366975060242 | 5749-308 |
| 00366975060235 | 5749-282 |
| 00366975060228 | 5749-273 |
| 00366975057570 | 5447-821 |
| 00366975057563 | 5447-812 |
| 00366975035882 | 5519-262 |
| 00366975035165 | 5495-547 |
| 00366975035158 | 5495-538 |
| 00366975035141 | 5495-529 |
| 00366975035134 | 5495-510 |
| 00366975010377 | 5220-726 |
| 00366975010353 | 5220-708 |
| 00366975010339 | 5220-824 |
| 00366975010322 | 5220-815 |
| 00366975010315 | 5220-806 |
| 00366975010308 | 5220-799 |
| 00366975010292 | 5220-780 |
| 00366975010285 | 5220-771 |
| 00366975010278 | 5220-762 |
| 00366975010261 | 5220-753 |
| 00366975010254 | 5220-744 |
| 00366975010247 | 5220-735 |
Trademark Results [VALULINE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
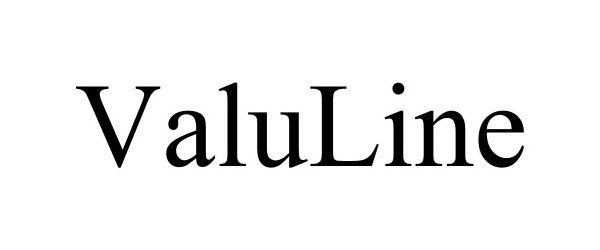 VALULINE 98406094 not registered Live/Pending |
Terra Universal, Inc. 2024-02-14 |
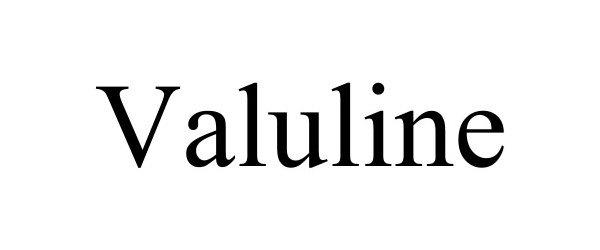 VALULINE 90830588 not registered Live/Pending |
Benco Dental Supply Co. 2021-07-15 |
 VALULINE 86713135 4922360 Live/Registered |
HYDROFARM, LLC 2015-08-03 |
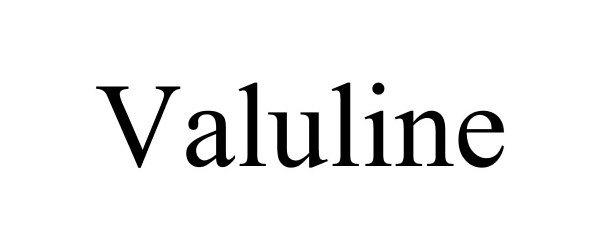 VALULINE 86148028 4657615 Live/Registered |
Milford Holding Company 2013-12-19 |
 VALULINE 77950389 3860385 Live/Registered |
COMMSCOPE TECHNOLOGIES LLC 2010-03-04 |
 VALULINE 77556476 3737803 Live/Registered |
Tarter Gate Company, LLC 2008-08-27 |
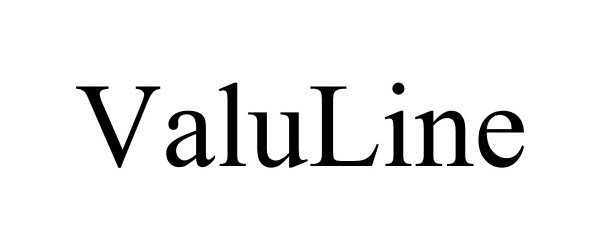 VALULINE 77367515 3633765 Dead/Cancelled |
HYDROFARM, INC. 2008-01-09 |
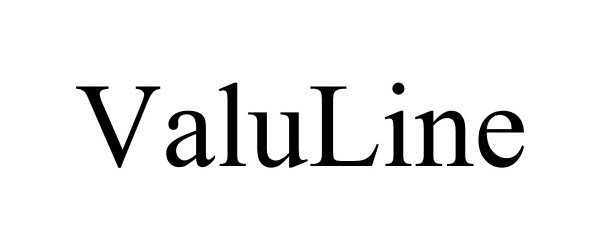 VALULINE 77367515 3633765 Dead/Cancelled |
Lovinger, Roberta 2008-01-09 |
 VALULINE 75872207 2742555 Dead/Cancelled |
Penn Champ, Inc. 1999-12-15 |
 VALULINE 75755783 not registered Dead/Abandoned |
BISSELL HOMECARE, INC. 1999-07-20 |
 VALULINE 74340489 1858127 Live/Registered |
COMMSCOPE TECHNOLOGIES LLC 1992-12-16 |
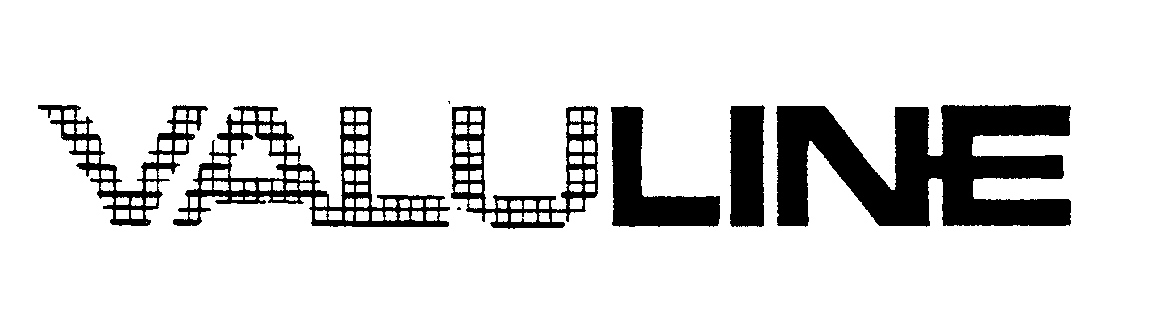 VALULINE 73515425 1350681 Dead/Cancelled |
STYLINE CORPORATION 1984-12-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.