Epix ® Inline Laparoscopic Dissector
GUDID 10607915124479
Disposable Dissector
APPLIED MEDICAL RESOURCES CORPORATION
Endoscopic electrosurgical handpiece/electrode, monopolar, single-use| Primary Device ID | 10607915124479 |
| NIH Device Record Key | 8ed26652-f3d8-4e72-b374-13c4eb540ec1 |
| Commercial Distribution Discontinuation | 2016-09-24 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Epix ® Inline Laparoscopic Dissector |
| Version Model Number | CY050 |
| Company DUNS | 187129135 |
| Company Name | APPLIED MEDICAL RESOURCES CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com | |
| Phone | 1.949.713.8300 |
| contact@appliedmedical.com |
Device Dimensions
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
| Length | 38 Centimeter |
| Outer Diameter | 5 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00607915124472 [Primary] |
| GS1 | 10607915124479 [Package] Contains: 00607915124472 Package: [10 Units] Discontinued: 2016-09-24 Not in Commercial Distribution |
FDA Product Code
| GEI | Electrosurgical, Cutting & Coagulation & Accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-02-10 |
| Device Publish Date | 2016-09-24 |
Devices Manufactured by APPLIED MEDICAL RESOURCES CORPORATION
| 00607915146467 - Voyant Electrosurgical Generator | 2026-01-26 |
| 00607915149024 - Inzii Ripstop Retrieval System | 2026-01-26 |
| 00607915140700 - Alexis® Orthopaedic Protector | 2025-04-16 X-S/M Flexible Alexis Orthopaedic Protector |
| 00607915114688 - Threaded Cannula and Seal | 2024-12-16 Laparoscopic Trocar Sleeve |
| 10607915140325 - Alexis® O Wound Protector-Retractor | 2024-09-04 Wound Retractor and Protector |
| 10607915145528 - Inzii Ripstop Redeployable Retrieval System | 2024-03-27 Redeployable tissue retrieval system with Ripstop nylon bag. |
| 00607915146498 - Suction Irrigation System | 2024-02-09 Probe and Valve with dual bag tubing. |
| 00607915146504 - Suction Irrigation System | 2024-02-09 Valve with dual bag tubing. |
Trademark Results [Epix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
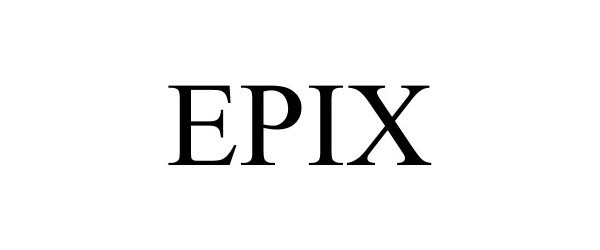 EPIX 90978897 not registered Live/Pending |
GW Research Limited 2021-01-18 |
 EPIX 90978896 not registered Live/Pending |
GW Research Limited 2021-01-18 |
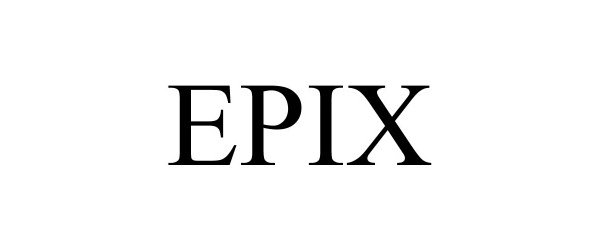 EPIX 90472235 not registered Live/Pending |
GW Research Limited 2021-01-18 |
 EPIX 90472233 not registered Live/Pending |
GW Research Limited 2021-01-18 |
 EPIX 88780889 not registered Live/Pending |
Sandals Resorts International 2000 Inc. 2020-01-31 |
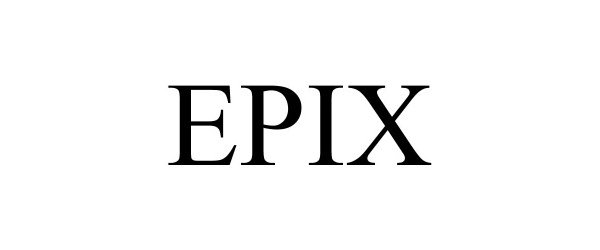 EPIX 88780872 not registered Live/Pending |
Sandals Resorts International 2000 Inc. 2020-01-31 |
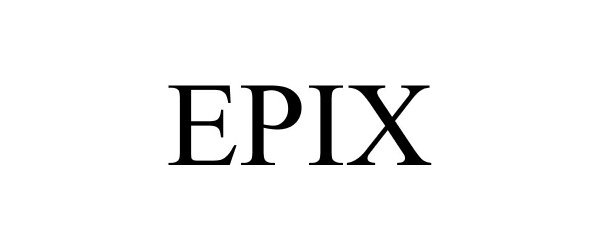 EPIX 88421137 not registered Live/Pending |
Keilhauer Ltd. 2019-05-08 |
 EPIX 88041166 not registered Live/Pending |
Henkel AG & Co. KGaA 2018-07-17 |
 EPIX 88041041 not registered Live/Pending |
Henkel AG & Co. KGaA 2018-07-17 |
 EPIX 87748180 not registered Live/Pending |
Encapsys, LLC 2018-01-09 |
 EPIX 87748113 not registered Live/Pending |
Encapsys, LLC 2018-01-09 |
 EPIX 87719523 5903858 Live/Registered |
Henkel AG & Co. KGaA 2017-12-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.