Tri-Grip™ Fixation Screw (2 pieces) Large Diameter (for Non-Engaging Abutments) 1000-52
GUDID 10841307109051
IMPLANT DIRECT SYBRON MANUFACTURING LLC
Dental implant suprastructure, permanent, preformed| Primary Device ID | 10841307109051 |
| NIH Device Record Key | 45b4b381-4fba-45e0-a81e-8389ca7209ae |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Tri-Grip™ Fixation Screw (2 pieces) Large Diameter (for Non-Engaging Abutments) |
| Version Model Number | 1000-52 |
| Catalog Number | 1000-52 |
| Company DUNS | 868856969 |
| Company Name | IMPLANT DIRECT SYBRON MANUFACTURING LLC |
| Device Count | 2 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00841307109054 [Unit of Use] |
| GS1 | 10841307109051 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K130572
FDA Product Code
| NHA | Abutment, Implant, Dental, Endosseous |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
[10841307109051]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-11-08 |
| Device Publish Date | 2015-09-14 |
On-Brand Devices [Tri-Grip™ Fixation Screw (2 pieces) Large Diameter (for Non-Engaging Abutments)]
| 10841307109082 | 1000-54 |
| 10841307109051 | 1000-52 |
Trademark Results [Tri-Grip]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
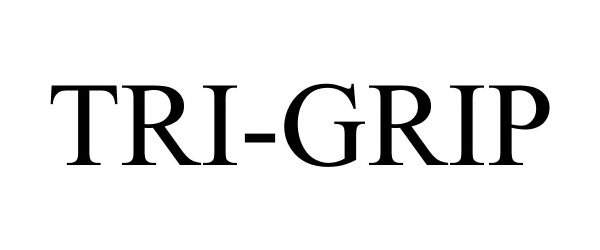 TRI-GRIP 87490432 not registered Live/Pending |
Cello Plastic Products Private Ltd 2017-06-15 |
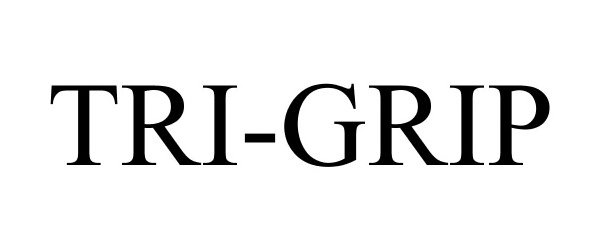 TRI-GRIP 87447637 5421979 Live/Registered |
Lexair, Inc. 2017-05-12 |
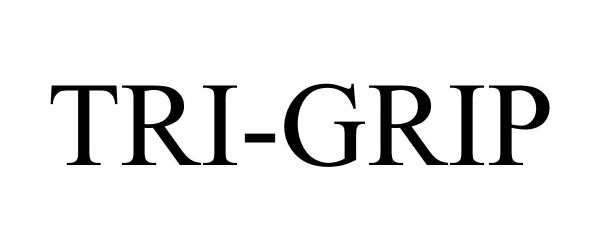 TRI-GRIP 86146958 4571959 Live/Registered |
10Back, LLC 2013-12-18 |
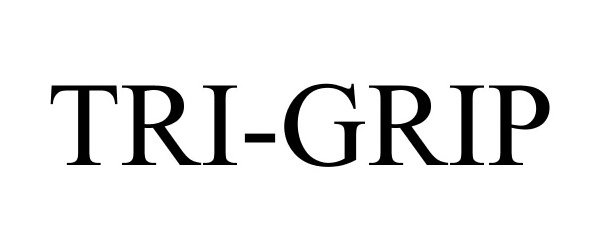 TRI-GRIP 77472778 3649594 Live/Registered |
TASCO CORPORATION 2008-05-13 |
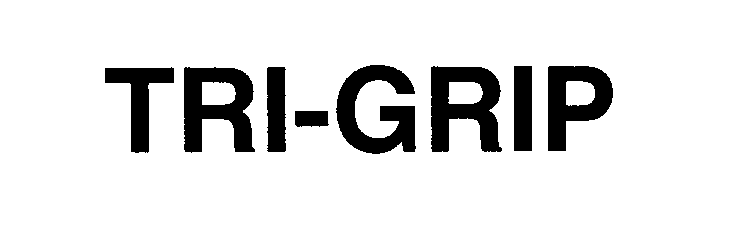 TRI-GRIP 76643128 3111060 Live/Registered |
Eckel Manufacturing Company, Inc. 2005-07-18 |
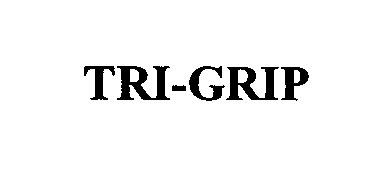 TRI-GRIP 76619393 not registered Dead/Abandoned |
CRI 2000, L.P. 2004-11-03 |
 TRI-GRIP 75327414 not registered Dead/Abandoned |
Collins, Stuart 1997-07-21 |
 TRI-GRIP 74476242 not registered Dead/Abandoned |
Itoya of America, Ltd. 1994-01-04 |
 TRI-GRIP 74420370 1847589 Dead/Cancelled |
GREENLEE TOOLS, INC. 1993-08-04 |
 TRI-GRIP 73828850 1596593 Dead/Cancelled |
ANDERSEN BROTHERS HOLDING COMPANY, INC., THE 1989-10-02 |
 TRI-GRIP 73081225 1048419 Dead/Cancelled |
W. W. Patterson Company 0000-00-00 |
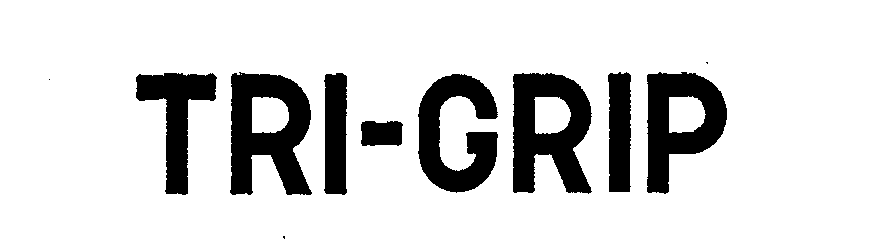 TRI-GRIP 72110469 0734073 Dead/Expired |
J. R. RICHARDS COMPANY 1960-12-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.