MULTICORE MLC2025
GUDID 18033959225940
Automatic guillotine type instrument for soft tissue biopsy 20G; 25 cm
STERYLAB SRL
Soft-tissue biopsy procedure kit, non-medicated| Primary Device ID | 18033959225940 |
| NIH Device Record Key | 1acfe74e-22c9-43c1-9f4f-be4f9e21f9d2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MULTICORE |
| Version Model Number | MULTICORE |
| Catalog Number | MLC2025 |
| Company DUNS | 439659574 |
| Company Name | STERYLAB SRL |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08033959225943 [Primary] |
| GS1 | 18033959225940 [Package] Contains: 08033959225943 Package: [15 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K051506
FDA Product Code
| KNW | Instrument, Biopsy |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-10-10 |
| Device Publish Date | 2019-10-02 |
On-Brand Devices [MULTICORE]
| 18033959225940 | Automatic guillotine type instrument for soft tissue biopsy 20G; 25 cm |
| 18033959225896 | Automatic guillotine type instrument for soft tissue biopsy 20G; 20 cm |
| 18033959225841 | Automatic guillotine type instrument for soft tissue biopsy 20G; 15 cm |
| 18033959225810 | Automatic guillotine type instrument for soft tissue biopsy 20G; 11 cm |
| 18033959225742 | Automatic guillotine type instrument for soft tissue biopsy 18G; 25cm |
| 18033959225667 | Automatic guillotine type instrument for soft tissue biopsy 18G; 20cm |
| 18033959225612 | Automatic guillotine type instrument for soft tissue biopsy 18G; 15 cm |
| 18033959225568 | Automatic guillotine type instrument for soft tissue biopsy 18G; 11 cm |
| 18033959225506 | Automatic guillotine type instrument for soft tissue biopsy 16G;25cm |
| 18033959225421 | Automatic guillotine type instrument for soft tissue biopsy 16G;20cm |
| 18033959225346 | Automatic guillotine type instrument for soft tissue biopsy 16G; 15 cm |
| 18033959225292 | Automatic guillotine type instrument for soft tissue biopsy 16G; 11 cm |
| 18033959225216 | Automatic guillotine type instrument for soft tissue biopsy 14G; 25 cm |
| 18033959225131 | Automatic guillotine type instrument for soft tissue biopsy 14G; 20 cm |
| 18033959225056 | Automatic guillotine type instrument for soft tissue biopsy 14G; 15cm |
| 18033959225001 | Automatic guillotine type instrument for soft tissue biopsy 14G; 11 cm |
Trademark Results [MULTICORE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
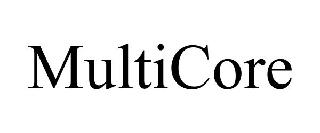 MULTICORE 98543281 not registered Live/Pending |
Guangdong Sweetnight Technical Innovation Co., Ltd. 2024-05-09 |
 MULTICORE 88044387 not registered Live/Pending |
Peregrine Turbine Technologies, LLC 2018-07-19 |
 MULTICORE 86347604 4695799 Live/Registered |
VITALIZE, LLC 2014-07-24 |
 MULTICORE 86013602 not registered Dead/Abandoned |
Henkel AG & Co. KGaA 2013-07-18 |
 MULTICORE 79024545 3261282 Live/Registered |
OCV Intellectual Capital, LLC 2005-07-01 |
 MULTICORE 77401278 3620493 Dead/Cancelled |
E. & O. MARI, INC. 2008-02-20 |
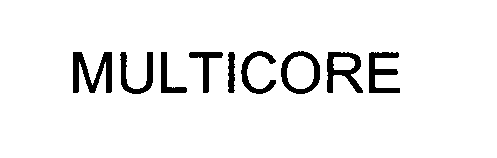 MULTICORE 76542287 2957758 Live/Registered |
Ivoclar Vivadent, Inc. 2003-09-03 |
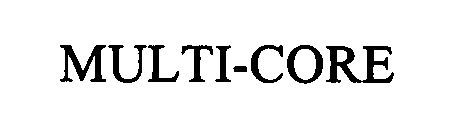 MULTICORE 76314280 2751086 Live/Registered |
BANOM, INC. 2001-09-19 |
 MULTICORE 73201560 1139385 Live/Registered |
Multicore Solders Limited 1979-01-25 |
 MULTICORE 73106761 1098385 Dead/Expired |
DART INDUSTRIES, INC. 1976-11-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.