EZ-Pak™ Kit
GUDID 20613994938484
CATHETER 388410 PF 5PK 6F EZ PK
MEDTRONIC, INC.
Angiographic catheter, single-use| Primary Device ID | 20613994938484 |
| NIH Device Record Key | e803b773-cbd8-4c4b-828d-e199cead2f28 |
| Commercial Distribution Discontinuation | 2018-10-05 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | EZ-Pak™ Kit |
| Version Model Number | 388410 |
| Company DUNS | 006261481 |
| Company Name | MEDTRONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com |
Device Dimensions
| Catheter Gauge | 6 French |
| Catheter Gauge | 6 French |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store catheters in a cool, dry area. |
| Special Storage Condition, Specify | Between 0 and 0 *Store catheters in a cool, dry area. |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00613994938480 [Primary] |
| GS1 | 20613994938484 [Package] Contains: 00613994938480 Package: PK [5 Units] Discontinued: 2018-10-05 Not in Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K960056
FDA Product Code
| DQO | CATHETER, INTRAVASCULAR, DIAGNOSTIC |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-01-15 |
| Device Publish Date | 2016-07-03 |
On-Brand Devices [EZ-Pak™ Kit]
| 20613994941200 | CATHETER 558500 PF 5PK KIT 6F BTO EZ PK |
| 20613994941163 | CATHETER 558453 PF 5PK KIT 7F ST EZ-PAK |
| 20613994940715 | CATHETER 558450 PF 5PK KIT 7F ST EZ-PAK |
| 20613994940692 | CATH 558448 PF 5PK KIT 6F ST XT EZPAK |
| 20613994940685 | CATHETER 558447 PF 5PK 6F ST EZ-PAK |
| 20613994940661 | CATH 558445 PF 5PK KIT 6F ST XT EZPAK |
| 20613994940654 | CATHETER 558443 PF 5PK 6F ST EZ-PAK |
| 20613994940630 | CATHETER 558420 PF 5PK 6F ST EZ-PAK |
| 20613994940623 | CATHETER 558419 PF 5PK 6F EZ PK |
| 20613994940616 | CATH 558418 PF 5PK KIT 6F ST XT EZPAK |
| 20613994940579 | CATHETER 554902 PF 5PK KIT 7F ST EZ-PAK |
| 20613994940302 | CATH 6A0107 SS 5PK6F EZ W/145 6SH.038INP |
| 20613994940227 | CATH 858448 PF 5PK KIT 6F ST XT EZPAK |
| 20613994940210 | CATHETER 858447 PF 5PK 6F ST EZ-PAK |
| 20613994940197 | CATH 858445 PF 5PK KIT 6F ST XT EZPAK |
| 20613994940180 | CATHETER 858443 PF 5PK 6F ST EZ-PAK |
| 20613994940166 | CATHETER 858420 PF 5PK 6F ST EZ-PAK |
| 20613994940159 | CATHETER 858419 PF 5PK 6F EZ PK |
| 20613994940142 | CATH 858418 PF 5PK KIT 6F ST XT EZPAK |
| 20613994939870 | CATH 6A0106 SS 5PK6F EZ W/145 6SH.035INP |
| 20613994939863 | CATH 6A0105 SS 5PK6F EZ W/STRXT 6SH .038 |
| 20613994939856 | CATH 6A0104 SS 5PK6F EZ W/STR XT 6SH.035 |
| 20613994939825 | CATH 6A0101 SS 5PK6F EZ W/145 PG.038 INP |
| 20613994939818 | CATH 6A0100 SS 5PK6F EZ W/.038 INP STRPG |
| 20613994939726 | CATH 6A0073 SS 5PK6F EZ W/3DRC XT.035INP |
| 20613994939702 | CATH 6A0068 SS 5PK6F EZ W/155 PG.35 INP |
| 20613994939696 | CATH 6A0067 SS 5PK6F EZ W/145 PG .035INP |
| 20613994939689 | CATH 6A0066 SS 5PK6F EZ W/STR PG .035INP |
| 20613994938569 | CATHETER 388420 PF 5PK 6F ST EZ-PAK |
| 20613994938484 | CATHETER 388410 PF 5PK 6F EZ PK |
| 20613994938422 | CATHETER 384902 PF 5PK KIT 7F ST EZ-PAK |
| 20613994938408 | CATH 358484 PF 5PK 6F BTO EZ W/.035 WIRE |
| 20613994938132 | CATHETER 350010 PF 5PK KIT 7F ST EZ-PAK |
| 20613994936220 | CATH 5A0093 SS 5PK5F EZ W/145 PG .038INP |
| 20613994936213 | CATH 5A0092 SS 5PK 5F EZ W/STR PG.038INP |
| 20613994936206 | CATH 5A0073 SS 5PK5F EZW/STR PG 3DRC INP |
| 20613994936183 | CATH 5A0067 SS 5PK 5F EZ W/155PTAIL INP |
| 20613994936176 | CATH 5A0066 SS 5PK 5F EZ W/145PTAIL INP |
| 20613994936169 | CATH 5A0065 SS 5PK 5F EZ W/INP STR PGT |
Trademark Results [EZ-Pak]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
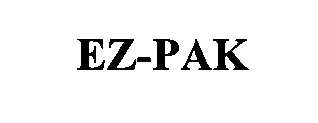 EZ-PAK 76434219 not registered Dead/Abandoned |
Harbison-Fischer, Inc. 2002-07-25 |
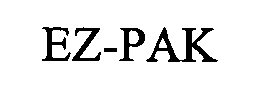 EZ-PAK 76331767 not registered Dead/Abandoned |
Pfizer Inc. 2001-10-30 |
 EZ-PAK 75503083 not registered Dead/Abandoned |
PAPER SYSTEMS, INC. 1998-06-16 |
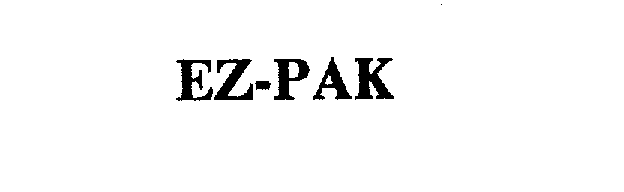 EZ-PAK 75041930 2348888 Live/Registered |
MERCK KGAA 1996-01-11 |
 EZ-PAK 74627507 1940093 Live/Registered |
YOUNG DENTAL MANUFACTURING I, LLC 1995-01-30 |
 EZ-PAK 74544169 1946411 Dead/Cancelled |
COMM/SCOPE, INC. 1994-06-28 |
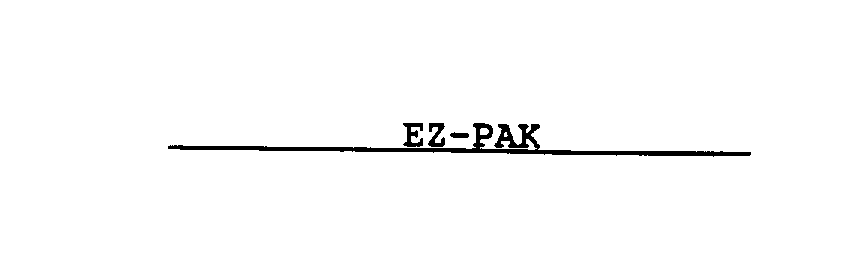 EZ-PAK 74467796 not registered Dead/Abandoned |
Moore Business Forms, Inc. 1993-12-09 |
 EZ-PAK 74463707 1918825 Dead/Cancelled |
Moore Business Forms, Inc. 1993-11-29 |
 EZ-PAK 74442437 1841766 Live/Registered |
MEDTRONIC VASCULAR, INC. 1993-09-30 |
 EZ-PAK 74189120 1697267 Dead/Cancelled |
Concrete Technology, Inc. 1991-07-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.