Shah
GUDID 20763000281834
VENT TUBE 1018101 5PK SHAH W/WIRE 1.14ID
MEDTRONIC XOMED, INC.
Tympanostomy tube| Primary Device ID | 20763000281834 |
| NIH Device Record Key | 7f3a802e-7925-4f18-b3d1-2be69190947b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Shah |
| Version Model Number | 1018101 |
| Company DUNS | 835465063 |
| Company Name | MEDTRONIC XOMED, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00763000281830 [Primary] |
| GS1 | 20763000281834 [Package] Contains: 00763000281830 Package: PK [5 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K151067
FDA Product Code
| ETD | TUBE, TYMPANOSTOMY |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-05-20 |
| Device Publish Date | 2024-05-10 |
On-Brand Devices [Shah]
| 20613994492559 | VENT TUBE MV16150 10PK SHAH FLPL |
| 20613994492542 | VENT TUBE 1018101 5PK SHAH W/WIRE 1.14ID |
| 20613994492535 | VENT TUBE 1018100 5PK SHAH 1.14ID FLPL |
| 20681490040888 | VENT TUBE 14311 5PK MINI SHAH .76MM FLPL |
| 20681490032180 | VENT TUBE 1056174 5PK SHAH 1.14 FLPL |
| 20763000038087 | VENT TUBE 1018100 5PK SHAH 1.14ID FLPL |
| 20763000039923 | VENT TUBE 14311 5PK MINI SHAH .76MM FLPL |
| 20763000038100 | VENT TUBE MV16150 10PK SHAH FLPL |
| 20763000038094 | VENT TUBE 1018101 5PK SHAH W/WIRE 1.14ID |
| 20763000281827 | VENT TUBE 1018100 5PK SHAH 1.14ID FLPL |
| 20763000282992 | VENT TUBE 14311 5PK MINI SHAH .76MM FLPL |
| 20763000281834 | VENT TUBE 1018101 5PK SHAH W/WIRE 1.14ID |
| 20763000870731 | VENT TUBE 14311 5PK MINI SHAH .76MM FLPL |
| 20763000870472 | VENT TUBE 1018101 5PK SHAH W/WIRE 1.14ID |
| 20763000870465 | VENT TUBE 1018100 5PK SHAH 1.14ID FLPL |
Trademark Results [Shah]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
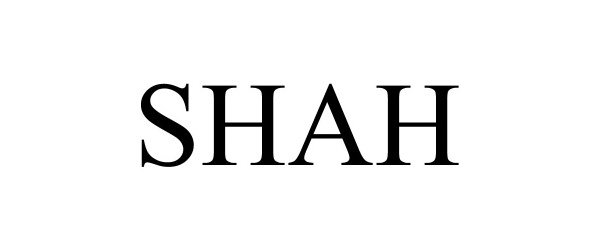 SHAH 97975808 not registered Live/Pending |
Shah Architecture, P.C. 2021-11-04 |
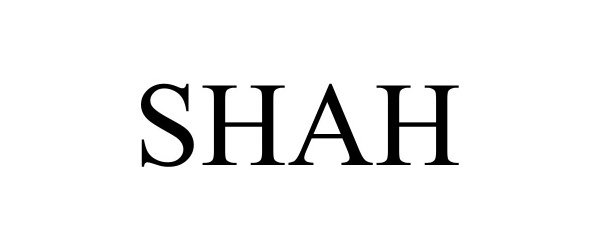 SHAH 97452352 not registered Live/Pending |
Shah Brands LLC 2022-06-10 |
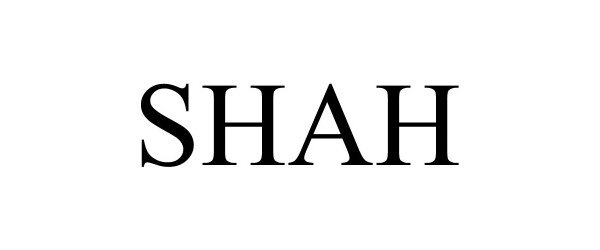 SHAH 97108940 not registered Live/Pending |
Shah Architecture, P.C. 2021-11-04 |
 SHAH 87154631 not registered Dead/Abandoned |
East Seventy Fourth Entertainment, LLC 2016-08-29 |
 SHAH 85673562 4371020 Live/Registered |
Kulwant Singh 2012-07-11 |
 SHAH 74383245 not registered Dead/Abandoned |
Blansh International 1993-04-27 |
 SHAH 73177357 1129924 Dead/Cancelled |
SHAH, JUDITH V. 1978-07-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.