Excite™ HPC201E/A
GUDID 20884450380905
Merit Medical Systems, Inc.
Radiographic procedure tubing| Primary Device ID | 20884450380905 |
| NIH Device Record Key | f9f8b441-1c6c-4633-a0fb-5500de729297 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Excite™ |
| Version Model Number | 00884450380901 |
| Catalog Number | HPC201E/A |
| Company DUNS | 184763290 |
| Company Name | Merit Medical Systems, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884450380901 [Primary] |
| GS1 | 10884450380908 [Package] Contains: 00884450380901 Package: [25 Units] In Commercial Distribution |
| GS1 | 20884450380905 [Package] Contains: 10884450380908 Package: [4 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K883718
FDA Product Code
| DXJ | DISPLAY, CATHODE-RAY TUBE, MEDICAL |
| FPK | Tubing, fluid delivery |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-02-10 |
| Device Publish Date | 2020-01-31 |
On-Brand Devices [Excite™]
| 10884450381004 | 00884450381007 |
| 10884450380991 | 00884450380994 |
| 20884450380981 | 00884450380987 |
| 10884450380977 | 00884450380970 |
| 20884450380950 | 00884450380956 |
| 10884450380939 | 00884450380932 |
| 20884450380912 | 00884450380918 |
| 10884450380892 | 00884450380895 |
| 10884450380885 | 00884450380888 |
| 10884450380878 | 00884450380871 |
| 20884450105256 | 00884450105252 |
| 10884450104344 | 00884450104347 |
| 10884450030346 | 00884450030349 |
| 10884450029234 | 00884450029237 |
| 20884450027947 | 00884450027943 |
| 10884450027933 | 00884450027936 |
| 10884450027926 | 00884450027929 |
| 10884450025663 | 00884450025666 |
| 20884450024267 | 00884450024263 |
| 10884450021429 | 00884450021422 |
| 20884450021419 | 00884450021415 |
| 20884450021402 | 00884450021408 |
| 10884450011437 | 00884450011430 |
| 20884450008571 | 00884450008577 |
| 10884450380946 | 00884450380949 |
| 20884450380929 | 00884450380925 |
| 20884450380905 | 00884450380901 |
Trademark Results [Excite]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
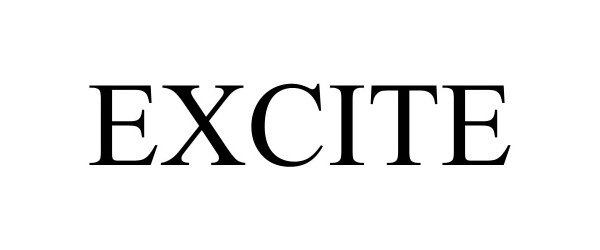 EXCITE 98512860 not registered Live/Pending |
Tire Group International, LLC. 2024-04-22 |
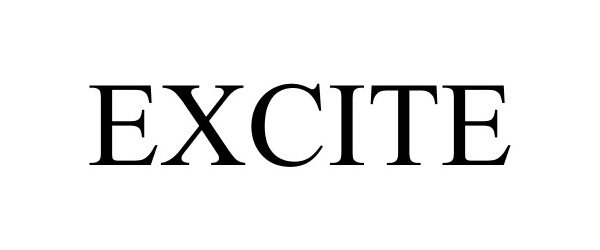 EXCITE 88237500 5886983 Live/Registered |
EXCITE CREDIT UNION 2018-12-20 |
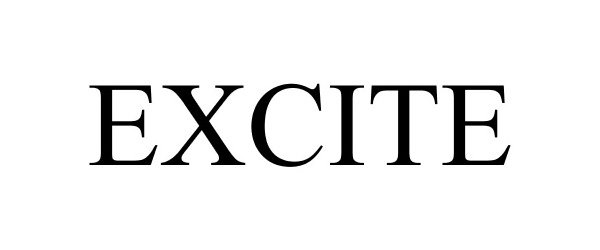 EXCITE 87958414 not registered Live/Pending |
Lifestyles Healthcare Pte Ltd 2018-06-12 |
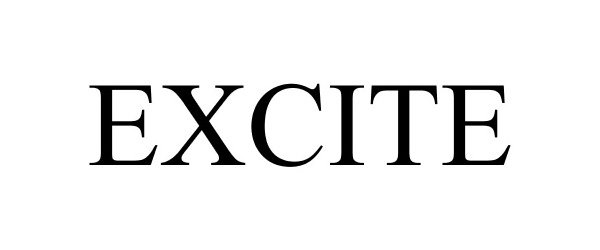 EXCITE 87345602 not registered Dead/Abandoned |
5 Dynamics, LLC 2017-02-22 |
 EXCITE 86028397 not registered Dead/Abandoned |
Novartis AG 2013-08-05 |
 EXCITE 85739284 not registered Dead/Abandoned |
Ansell Limited 2012-09-26 |
 EXCITE 85722455 4292472 Live/Registered |
LEIDOS INNOVATIONS TECHNOLOGY, INC. 2012-09-06 |
 EXCITE 85722421 4382734 Live/Registered |
LEIDOS INNOVATIONS TECHNOLOGY, INC. 2012-09-06 |
 EXCITE 85624431 4356621 Live/Registered |
Crestron Electronics, Inc. 2012-05-14 |
 EXCITE 85471416 4433074 Live/Registered |
JUKI Corporation 2011-11-14 |
 EXCITE 85386996 4199884 Live/Registered |
DYNABOOK AMERICAS, INC. 2011-08-02 |
 EXCITE 85054043 not registered Dead/Abandoned |
Crestron Electronics, Inc. 2010-06-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.