MoMe® ARC
GUDID B690MA20
The MoMe ARC® device consists of a Sensor Pod, Leads, Gateway, and Charging Cradle with accessories. The body worn Sensor Pod acquires, stores and forwards electrocardiogram (ECG) data from Leads in a Wired Lead-Set or in a Patch to the Gateway using a 2.4GHz BLE wireless link. The Gateway consists of an OTS mobile device running the MoMe ARC® Gateway Mobile App. The Gateway is a Medical Device Data System (MDDS) which stores and forwards the ECG signal data to the MoMe Software Platform (K152491) via a wireless cellular link. The MoMe ARC® communicates with the MoMe Software Platform (K152491), a web-based remote server software with proprietary algorithms for analysis, using the MoMe Device Communications Protocol. The MoMe Software System (K152491) analyzes the data via the embedded algorithm and, when indicated, data identified by the algorithm is flagged for physician review. Once activated and operating normally, the system requires no patient intervention to capture or analyze data. However, the MoMe ARC® has an optional patient triggered event feature that allows for manual selection and recording of patient symptoms, if and when desired. The device is intended for use under prescription only (Rx only) for monitoring patients with suspected cardiac arrhythmias.
Infobionic, Inc.
Patient monitoring system module, electrocardiographic, telemetric| Primary Device ID | B690MA20 |
| NIH Device Record Key | eba7ae59-31d1-48ab-becf-c624c40b54ba |
| Commercial Distribution Discontinuation | 2031-08-25 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MoMe® ARC |
| Version Model Number | 32000 |
| Company DUNS | 078650138 |
| Company Name | Infobionic, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com | |
| Phone | 707-694-4905 |
| lleal@infobionic.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
| Storage Environment Temperature | Between 5 Degrees Celsius and 50 Degrees Celsius |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 93 Percent (%) Relative Humidity |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B690MA20 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K250356
FDA Product Code
| DSI | Detector And Alarm, Arrhythmia |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-09-02 |
| Device Publish Date | 2025-08-25 |
Devices Manufactured by Infobionic, Inc.
| B690MA20 - MoMe® ARC | 2025-09-02The MoMe ARC® device consists of a Sensor Pod, Leads, Gateway, and Charging Cradle with accessories. The body worn Sensor Pod acquires, stores and forwards electrocardiogram (ECG) data from Leads in a Wired Lead-Set or in a Patch to the Gateway using a 2.4GHz BLE wireless link. The Gateway consists of an OTS mobile device running the MoMe ARC® Gateway Mobile App. The Gateway is a Medical Device Data System (MDDS) which stores and forwards the ECG signal data to the MoMe Software Platform (K152491) via a wireless cellular link. The MoMe ARC® communicates with the MoMe Software Platform (K152491), a web-based remote server software with proprietary algorithms for analysis, using the MoMe Device Communications Protocol. The MoMe Software System (K152491) analyzes the data via the embedded algorithm and, when indicated, data identified by the algorithm is flagged for physician review. Once activated and operating normally, the system requires no patient intervention to capture or analyze data. However, the MoMe ARC® has an optional patient triggered event feature that allows for manual selection and recording of patient symptoms, if and when desired. The device is intended for use under prescription only (Rx only) for monitoring patients with suspected cardiac arrhythmias. |
| B690MA20 - MoMe® ARC | 2025-09-02 The MoMe ARC® device consists of a Sensor Pod, Leads, Gateway, and Charging Cradle with accessories. The body worn Sensor Pod a |
| B690MA10 - MoMe® ARC | 2024-01-05 MoMe® ARC is a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in patie |
| B690MK10 - MoMe® Kardia | 2019-05-07 MoMe® Kardia is a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in pa |
| B690MS10 - MoMe® Software Platform | 2018-10-24 MoMe® Software Platform is a web-based remote server software with proprietary algorithms for analysis of ECG data. It analyzes |
Trademark Results [MoMe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
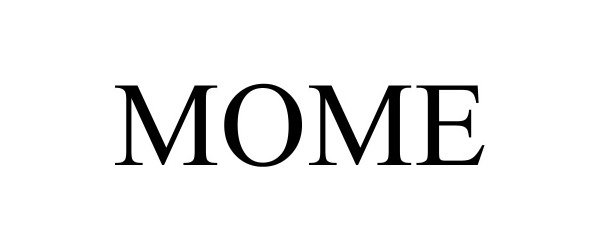 MOME 98403977 not registered Live/Pending |
Keystone Robotix LLC 2024-02-13 |
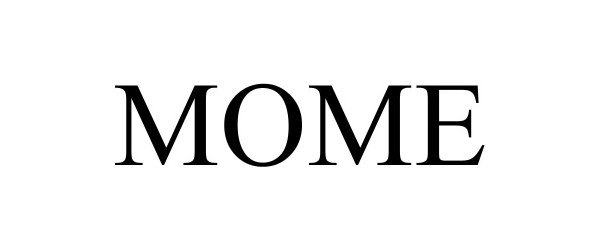 MOME 98279337 not registered Live/Pending |
Rodas, Gloria 2023-11-21 |
 MOME 97340220 not registered Live/Pending |
Mome Entertainment Inc. 2022-03-31 |
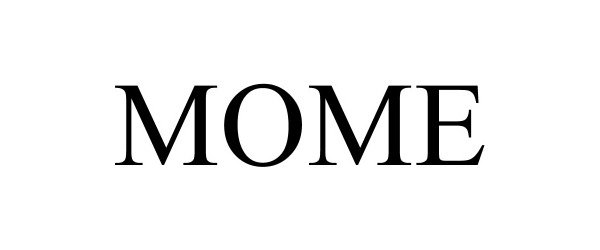 MOME 97314019 not registered Live/Pending |
Alive Network Pty Ltd. 2022-03-15 |
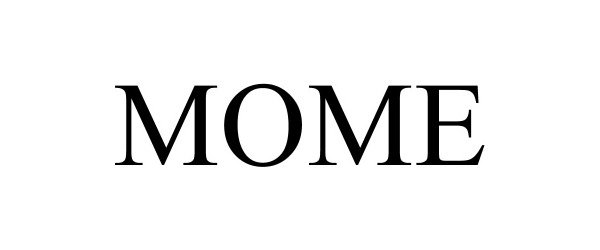 MOME 97103328 not registered Live/Pending |
Alive Network Pty Ltd. 2021-11-01 |
 MOME 90331666 not registered Live/Pending |
Brace, Robert 2020-11-20 |
 MOME 87546046 5346469 Live/Registered |
Ashok Mewani 2017-07-27 |
 MOME 85612067 4667959 Live/Registered |
InfoBionic, Inc. 2012-04-30 |
 MOME 85195284 not registered Dead/Abandoned |
Mewani Ashok 2010-12-10 |
 MOME 77738836 3883697 Dead/Cancelled |
Mewani, Ashok 2009-05-17 |
 MOME 73544459 1403836 Dead/Cancelled |
INTERNATIONAL SOFTWARE TECHNOLOGY, INC. 1985-06-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.