MoMe® Kardia
GUDID B690MK10
MoMe® Kardia is a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in patients with a demonstrated need for cardiac monitoring. MoMe® Kardia includes the wearable device that acquires and stores ECG data and transmits that data via cellular technology to the MoMe® Software Platform, a web-based remote server software with proprietary algorithms for analysis. MoMe® Software Platform analyzes the data via the embedded algorithm and when indicated, data identified by the algorithm is flagged for physician review.
INFOBIONIC, INC.
Electrocardiography telemetric monitoring system transmitter| Primary Device ID | B690MK10 |
| NIH Device Record Key | eddf3e53-8cc4-4f53-96f7-f21aa96a65b4 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MoMe® Kardia |
| Version Model Number | 01854 |
| Company DUNS | 078650138 |
| Company Name | INFOBIONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | B690MK10 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K160064
FDA Product Code
| DSI | Detector And Alarm, Arrhythmia |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-05-07 |
| Device Publish Date | 2018-09-23 |
Devices Manufactured by INFOBIONIC, INC.
| B690MA20 - MoMe® ARC | 2025-09-02 The MoMe ARC® device consists of a Sensor Pod, Leads, Gateway, and Charging Cradle with accessories. The body worn Sensor Pod a |
| B690MA10 - MoMe® ARC | 2024-01-05 MoMe® ARC is a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in patie |
| B690MK10 - MoMe® Kardia | 2019-05-07MoMe® Kardia is a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in patients with a demonstrated need for cardiac monitoring. MoMe® Kardia includes the wearable device that acquires and stores ECG data and transmits that data via cellular technology to the MoMe® Software Platform, a web-based remote server software with proprietary algorithms for analysis. MoMe® Software Platform analyzes the data via the embedded algorithm and when indicated, data identified by the algorithm is flagged for physician review. |
| B690MK10 - MoMe® Kardia | 2019-05-07 MoMe® Kardia is a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in pa |
| B690MS10 - MoMe® Software Platform | 2018-10-24 MoMe® Software Platform is a web-based remote server software with proprietary algorithms for analysis of ECG data. It analyzes |
Trademark Results [MoMe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
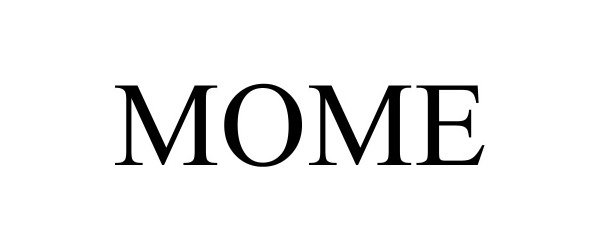 MOME 98403977 not registered Live/Pending |
Keystone Robotix LLC 2024-02-13 |
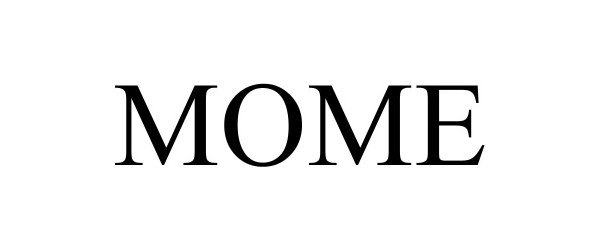 MOME 98279337 not registered Live/Pending |
Rodas, Gloria 2023-11-21 |
 MOME 97340220 not registered Live/Pending |
Mome Entertainment Inc. 2022-03-31 |
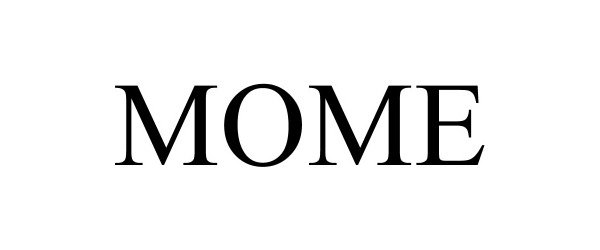 MOME 97314019 not registered Live/Pending |
Alive Network Pty Ltd. 2022-03-15 |
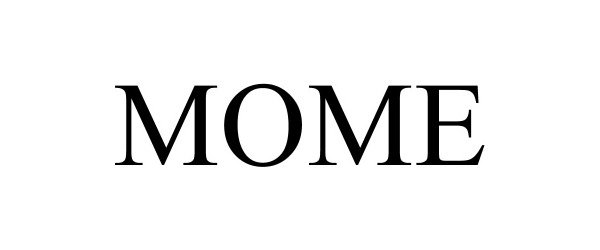 MOME 97103328 not registered Live/Pending |
Alive Network Pty Ltd. 2021-11-01 |
 MOME 90331666 not registered Live/Pending |
Brace, Robert 2020-11-20 |
 MOME 87546046 5346469 Live/Registered |
Ashok Mewani 2017-07-27 |
 MOME 85612067 4667959 Live/Registered |
InfoBionic, Inc. 2012-04-30 |
 MOME 85195284 not registered Dead/Abandoned |
Mewani Ashok 2010-12-10 |
 MOME 77738836 3883697 Dead/Cancelled |
Mewani, Ashok 2009-05-17 |
 MOME 73544459 1403836 Dead/Cancelled |
INTERNATIONAL SOFTWARE TECHNOLOGY, INC. 1985-06-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.