NYLON
GUDID E203US51540
Resorba Medical GmbH
Nylon suture, non-bioabsorbable, monofilament| Primary Device ID | E203US51540 |
| NIH Device Record Key | 99508283-a429-42bd-8bf4-bc059f4f215c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | NYLON |
| Version Model Number | NYLON |
| Company DUNS | 341053195 |
| Company Name | Resorba Medical GmbH |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| HIBCC | E203US51540 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K143582
FDA Product Code
| GAR | Suture, Nonabsorbable, Synthetic, Polyamide |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-04-08 |
| Device Publish Date | 2019-01-30 |
On-Brand Devices [NYLON]
| E203US5157 | NYLON |
| E203US5156 | NYLON |
| E203US51540 | NYLON |
| E203US51523 | NYLON |
| E203US51520 | NYLON |
| E203US51519 | NYLON |
| E203US51518 | NYLON |
| E203US51517 | NYLON |
| E203US51516 | NYLON |
| E203US51515 | NYLON |
| E203US51514 | NYLON |
| E203US51513 | NYLON |
| E203US5148 | NYLON |
| E203US5147 | NYLON |
| E203US5146 | NYLON |
| E203US5145 | NYLON |
| E203US5144 | NYLON |
| E203US51439 | NYLON |
| E203US5143 | NYLON |
| E203US51419 | NYLON |
| E203US51416 | NYLON |
| E203US51415 | NYLON |
| E203US5116 | NYLON |
| E203US5149 | NYLON |
| E203US5061 | NYLON |
| E203US51539 | NYLON |
| E203US51442 | - |
Trademark Results [NYLON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
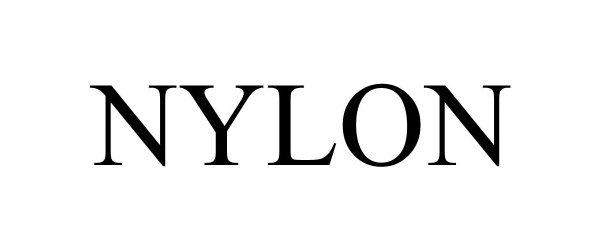 NYLON 98094638 not registered Live/Pending |
BDG Media, Inc. 2023-07-20 |
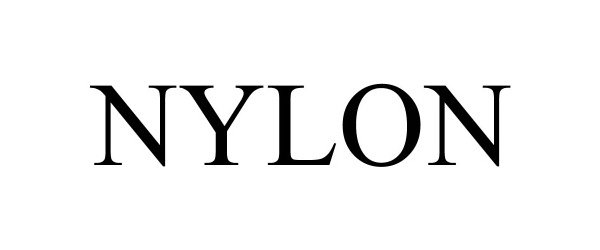 NYLON 90453767 not registered Live/Pending |
BDG Media, Inc. 2021-01-07 |
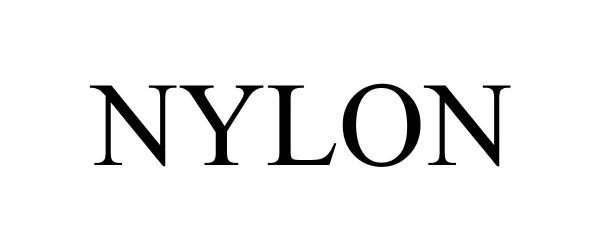 NYLON 85885426 4668157 Live/Registered |
Nylon Media, Inc. 2013-03-25 |
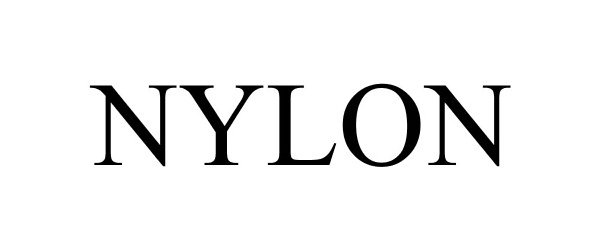 NYLON 85885418 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885412 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885407 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885403 4672358 Live/Registered |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 85885394 not registered Dead/Abandoned |
NYLON MEDIA, INC. 2013-03-25 |
 NYLON 79052496 3661040 Dead/Cancelled |
Nylon Studios Pty Limited 2008-03-04 |
 NYLON 77405209 3601225 Live/Registered |
BDG Media, Inc. 2008-02-25 |
 NYLON 76367518 not registered Dead/Abandoned |
Brunetti, Mary J. 2002-02-06 |
 NYLON 75942735 not registered Dead/Abandoned |
Nylon, L.L.C. 2000-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.