NDC 35781-0194
TRANZGEL
Echinacea Angustifolia, Echinacea Purpurea, Aconitum Napellus, Arnica Montana, Calendula Officianalis, Hamamelis Virginiana, Belladonna, Bellis Perennis, Chamomillia, Millefolium, Hypericum Perforatum
TRANZGEL is a Transdermal Gel in the Human Prescription Drug category. It is labeled and distributed by Gensco Laboratories, Llc. The primary component is Echinacea Angustifolia; Echinacea Purpurea; Aconitum Napellus; Arnica Montana; Calendula Officinalis Flowering Top; Hamamelis Virginiana Root Bark/stem Bark; Atropa Belladonna; Bellis Perennis; Chamomile; Achillea Millefolium; Hypericum Oil; Comfrey Root; Colchicine.
| Product ID | 35781-0194_9135b37f-d8f4-44ae-a7b3-677a1c83ee84 |
| NDC | 35781-0194 |
| Product Type | Human Prescription Drug |
| Proprietary Name | TRANZGEL |
| Generic Name | Echinacea Angustifolia, Echinacea Purpurea, Aconitum Napellus, Arnica Montana, Calendula Officianalis, Hamamelis Virginiana, Belladonna, Bellis Perennis, Chamomillia, Millefolium, Hypericum Perforatum |
| Dosage Form | Gel |
| Route of Administration | TRANSDERMAL |
| Marketing Start Date | 2010-07-01 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | Gensco Laboratories, LLC |
| Substance Name | ECHINACEA ANGUSTIFOLIA; ECHINACEA PURPUREA; ACONITUM NAPELLUS; ARNICA MONTANA; CALENDULA OFFICINALIS FLOWERING TOP; HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK; ATROPA BELLADONNA; BELLIS PERENNIS; CHAMOMILE; ACHILLEA MILLEFOLIUM; HYPERICUM OIL; COMFREY ROOT; COLCHICINE |
| Active Ingredient Strength | 1 [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL; [hp_X]/50mL |
| Pharm Classes | Cytochrome P450 3A4 Inhibitors [MoA],P-Glycoprotein Interactions [MoA] |
| NDC Exclude Flag | E |
| Listing Certified Through | 2018-12-31 |
Packaging
NDC 35781-0194-5
1 BOTTLE, DISPENSING in 1 CARTON (35781-0194-5) > 50 mL in 1 BOTTLE, DISPENSING
| Marketing Start Date | 2010-07-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 35781-0194-5 [35781019405]
TRANZGEL GEL
| Marketing Category | unapproved homeopathic |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | ML |
| Marketing Start Date | 2010-07-01 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ECHINACEA ANGUSTIFOLIA | 1 [hp_X]/50mL |
OpenFDA Data
| SPL SET ID: | abc4b131-4d35-46a4-9a8e-9bd4c7a0f4b0 |
| Manufacturer | |
| UNII |
Pharmacological Class
- Cytochrome P450 3A4 Inhibitors [MoA]
- P-Glycoprotein Interactions [MoA]
NDC Crossover Matching brand name "TRANZGEL" or generic name "Echinacea Angustifolia, Echinacea Purpurea, Aconitum Napellus, Arnica Montana, Calendula Officianalis, Hamamelis Virginiana, Belladonna, Bellis Perennis, Chamomillia, Millefolium, Hypericum Perforatum"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 35781-0194 | TRANZGEL | ECHINACEA ANGUSTIFOLIA, ECHINACEA PURPUREA, ACONITUM NAPELLUS, ARNICA MONTANA, CALENDULA OFFICIANALIS, HAMAMELIS VIRGINIANA, BELLADONNA, BELLIS PERENNIS, CHAMOMILLIA, MILLEFOLIUM, HYPERICUM PERFORATUM |
| 50436-9071 | TRANZGEL | ECHINACEA ANGUSTIFOLIA, ECHINACEA PURPUREA, ACONITUM NAPELLUS, ARNICA MONTANA, CALENDULA OFFICIANALIS, HAMAMELIS VIRGINIANA, BELLADONNA, BELLIS PERENNIS, CHAMOMILLIA, MILLEFOLIUM, HYPERICUM PERFORATUM, SYMPHYTUM OFFICINALE, COLCHICINUM |
Trademark Results [TRANZGEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
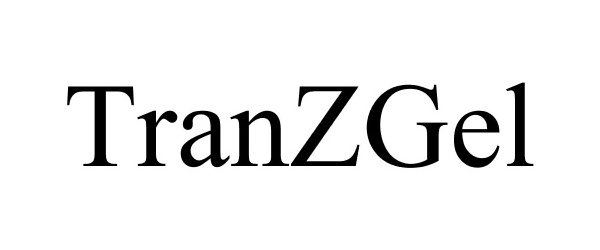 TRANZGEL 77741930 4168267 Live/Registered |
GENSCO LABORATORIES, LLC 2009-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.