NDC 36987-1734
Sage
Sage
Sage is a Intradermal; Subcutaneous Injection, Solution in the Human Prescription Drug category. It is labeled and distributed by Nelco Laboratories, Inc.. The primary component is Salvia Officinalis.
| Product ID | 36987-1734_dec580ea-73db-4658-9de1-440c19bb42b7 |
| NDC | 36987-1734 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Sage |
| Generic Name | Sage |
| Dosage Form | Injection, Solution |
| Route of Administration | INTRADERMAL; SUBCUTANEOUS |
| Marketing Start Date | 1972-08-29 |
| Marketing Category | BLA / BLA |
| Application Number | BLA102192 |
| Labeler Name | Nelco Laboratories, Inc. |
| Substance Name | SALVIA OFFICINALIS |
| Active Ingredient Strength | 0 g/mL |
| Pharm Classes | Non-Standardized Plant Allergenic Extract [EPC],Non-Standardized Food Allergenic Extract [EPC],Increased Histamine Release [PE],Cell-mediated Immunity [PE],Allergens [CS],Dietary Proteins [CS],Plant Proteins [CS] |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 36987-1734-2
10 mL in 1 VIAL, MULTI-DOSE (36987-1734-2)
| Marketing Start Date | 1972-08-29 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 36987-1734-1 [36987173401]
Sage INJECTION, SOLUTION
| Marketing Category | BLA |
| Application Number | BLA102192 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1972-08-29 |
| Inactivation Date | 2019-10-21 |
NDC 36987-1734-4 [36987173404]
Sage INJECTION, SOLUTION
| Marketing Category | BLA |
| Application Number | BLA102192 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1972-08-29 |
| Inactivation Date | 2019-10-21 |
NDC 36987-1734-2 [36987173402]
Sage INJECTION, SOLUTION
| Marketing Category | BLA |
| Application Number | BLA102192 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1972-08-29 |
| Inactivation Date | 2019-10-21 |
NDC 36987-1734-3 [36987173403]
Sage INJECTION, SOLUTION
| Marketing Category | BLA |
| Application Number | BLA102192 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1972-08-29 |
| Inactivation Date | 2019-10-21 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| SALVIA OFFICINALIS | .05 g/mL |
OpenFDA Data
Pharmacological Class
- Non-Standardized Plant Allergenic Extract [EPC]
- Non-Standardized Food Allergenic Extract [EPC]
- Increased Histamine Release [PE]
- Cell-mediated Immunity [PE]
- Allergens [CS]
- Dietary Proteins [CS]
- Plant Proteins [CS]
NDC Crossover Matching brand name "Sage" or generic name "Sage"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 36987-1734 | Sage | Sage |
| 36987-1735 | Sage | Sage |
| 36987-1736 | Sage | Sage |
| 36987-1737 | Sage | Sage |
| 49288-0493 | Sage | Sage |
| 54575-454 | SAGE FOOD | sage |
| 68428-617 | Salvia officinalis | SAGE |
| 71919-604 | Salvia officinalis | SAGE |
| 13838-1428 | Salvia officinalis (N) | SAGE |
Trademark Results [Sage]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SAGE 98732322 not registered Live/Pending |
Savant Systems, Inc. 2024-09-04 |
 SAGE 98709556 not registered Live/Pending |
Savant Systems, Inc. 2024-08-21 |
 SAGE 98624123 not registered Live/Pending |
Sage Global Services Limited 2024-06-28 |
 SAGE 98624111 not registered Live/Pending |
Sage Global Services Limited 2024-06-28 |
 SAGE 98606828 not registered Live/Pending |
SAGE DESIGNS LLC 2024-06-18 |
 SAGE 98484579 not registered Live/Pending |
Stylecraft, LLC 2024-04-04 |
 SAGE 98358492 not registered Live/Pending |
Shanghai Sage Intelligent Technology Co., Ltd. 2024-01-16 |
 SAGE 98244245 not registered Live/Pending |
Perfeggs Technology Co., Limited 2023-10-27 |
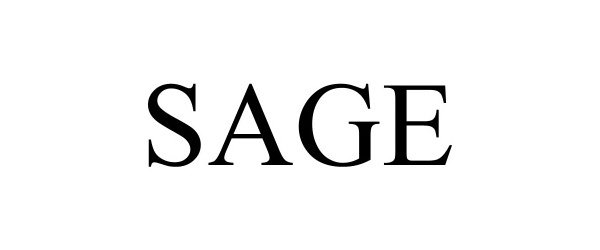 SAGE 98186128 not registered Live/Pending |
Sage Social LLC 2023-09-19 |
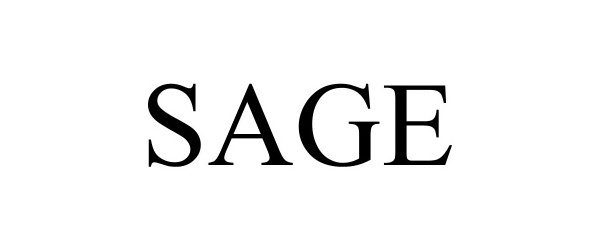 SAGE 98183960 not registered Live/Pending |
Sage Sustainable Electronics, LLC 2023-09-18 |
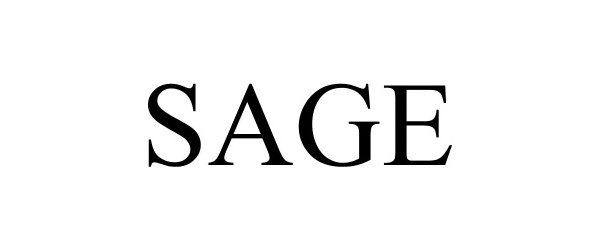 SAGE 98146609 not registered Live/Pending |
PEG Companies, Inc 2023-08-23 |
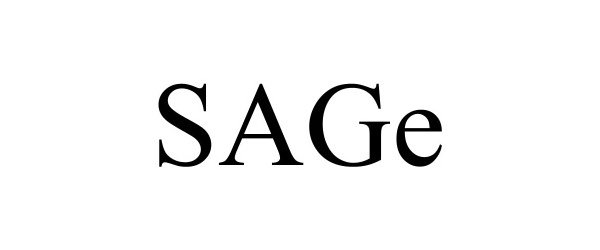 SAGE 98082350 not registered Live/Pending |
Reinisch, Mark E 2023-07-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.