NDC 50633-910
ThyroSafe
Potassium Iodide
ThyroSafe is a Oral Tablet in the Human Otc Drug category. It is labeled and distributed by Btg International Inc.. The primary component is Potassium Iodide.
| Product ID | 50633-910_ae74f926-f0a4-4dd6-9dfb-79fd8454cac2 |
| NDC | 50633-910 |
| Product Type | Human Otc Drug |
| Proprietary Name | ThyroSafe |
| Generic Name | Potassium Iodide |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2002-09-30 |
| Marketing Category | ANDA / |
| Application Number | ANDA076350 |
| Labeler Name | BTG International Inc. |
| Substance Name | POTASSIUM IODIDE |
| Active Ingredient Strength | 65 mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 50633-910-20
160 BOX in 1 CARTON (50633-910-20) > 20 BLISTER PACK in 1 BOX > 1 TABLET in 1 BLISTER PACK
| Marketing Start Date | 2002-09-30 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "ThyroSafe" or generic name "Potassium Iodide"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 50633-910 | ThyroSafe | Potassium Iodide |
| 66983-350 | ThyroSafe | Potassium Iodide |
| 51803-001 | IOSAT | Potassium Iodide |
| 51803-002 | IOSAT | Potassium Iodide |
| 0220-2798 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2799 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2800 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2801 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2802 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2803 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2804 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2805 | Kali iodatum | POTASSIUM IODIDE |
| 0220-2887 | Kali iodatum | POTASSIUM IODIDE |
| 10191-1857 | KALI IODATUM | POTASSIUM IODIDE |
| 68428-453 | Kali iodatum | POTASSIUM IODIDE |
| 71919-383 | Kali iodatum | POTASSIUM IODIDE |
| 43406-0094 | KALI IODATUM 1X | Potassium Iodide |
| 0178-0314 | POTASSIUM IODIDE | POTASSIUM IODIDE |
| 16477-601 | Potassium Iodide | Potassium Iodide |
| 75834-280 | Potassium Iodide | Potassium Iodide |
| 0245-0003 | SSKI | Potassium Iodide |
| 17856-0114 | SSKI | Potassium Iodide |
| 71740-112 | SSKI | Potassium Iodide |
| 0256-0210 | ThyroShield | Potassium Iodide |
| 59465-210 | ThyroShield | Potassium Iodide |
Trademark Results [ThyroSafe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
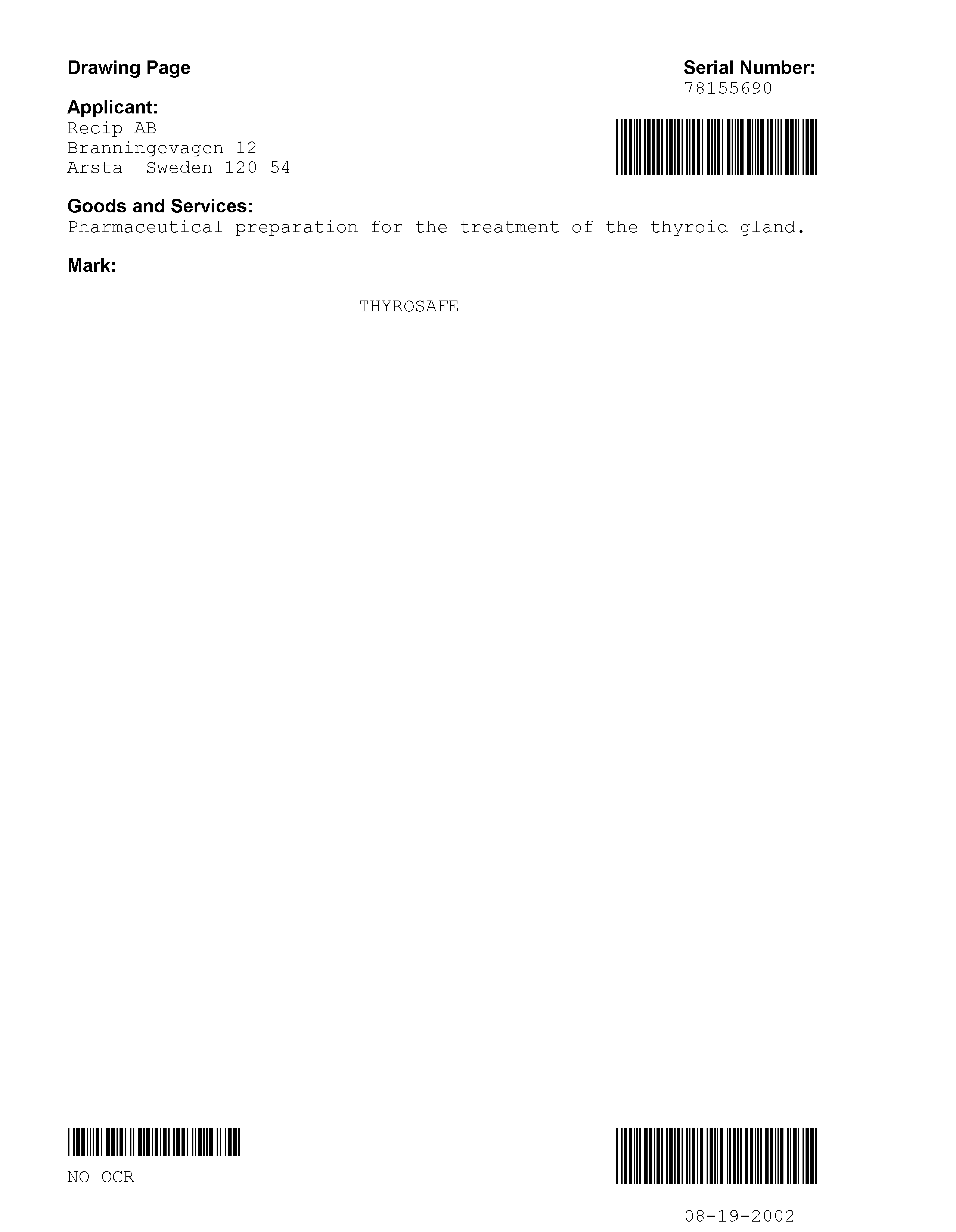 THYROSAFE 78155690 2826497 Live/Registered |
RPH PHARMACEUTICALS AB 2002-08-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.