NDC 52565-093
AquaMEPHYTON
Phytonadione
AquaMEPHYTON is a Intramuscular; Intravenous; Subcutaneous Injection, Emulsion in the Human Prescription Drug category. It is labeled and distributed by Teligent Pharma, Inc.. The primary component is Phytonadione.
| Product ID | 52565-093_2eb2025f-7602-4f3c-be99-547033e04ad7 |
| NDC | 52565-093 |
| Product Type | Human Prescription Drug |
| Proprietary Name | AquaMEPHYTON |
| Generic Name | Phytonadione |
| Dosage Form | Injection, Emulsion |
| Route of Administration | INTRAMUSCULAR; INTRAVENOUS; SUBCUTANEOUS |
| Marketing Start Date | 2018-03-07 |
| Marketing Category | NDA / NDA |
| Application Number | NDA012223 |
| Labeler Name | Teligent Pharma, Inc. |
| Substance Name | PHYTONADIONE |
| Active Ingredient Strength | 10 mg/mL |
| Pharm Classes | Increased Prothrombin Activity [PE],Reversed Anticoagulation Activity [PE],Vitamin K [CS],Vitamin K [EPC],Warfarin Reversal Agent [EPC] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 52565-093-05
5 AMPULE in 1 TRAY (52565-093-05) > 1 mL in 1 AMPULE
| Marketing Start Date | 2018-03-07 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 52565-093-01 [52565009301]
AquaMEPHYTON INJECTION, EMULSION
| Marketing Category | NDA |
| Application Number | NDA012223 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2017-05-25 |
| Marketing End Date | 2019-02-22 |
NDC 52565-093-05 [52565009305]
AquaMEPHYTON INJECTION, EMULSION
| Marketing Category | NDA |
| Application Number | NDA012223 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2018-03-07 |
| Marketing End Date | 2019-10-17 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| PHYTONADIONE | 10 mg/mL |
OpenFDA Data
| SPL SET ID: | 202757c7-caf6-44f5-9701-b89e4871df59 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Increased Prothrombin Activity [PE]
- Reversed Anticoagulation Activity [PE]
- Vitamin K [CS]
- Vitamin K [EPC]
- Warfarin Reversal Agent [EPC]
NDC Crossover Matching brand name "AquaMEPHYTON" or generic name "Phytonadione"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 52565-092 | AquaMEPHYTON | phytonadione |
| 52565-093 | AquaMEPHYTON | PHYTONADIONE |
| 0187-1704 | Mephyton | phytonadione |
| 21695-168 | MEPHYTON | phytonadione |
| 50090-1753 | Mephyton | phytonadione |
| 0404-9935 | PHYTONADIONE | phytonadione |
| 0404-9969 | PHYTONADIONE | phytonadione |
| 0904-6882 | Phytonadione | Phytonadione |
| 16714-973 | Phytonadione | Phytonadione |
| 43598-405 | PHYTONADIONE | PHYTONADIONE |
| 50268-661 | Phytonadione | phytonadione |
| 52565-142 | Phytonadione | phytonadione |
| 52565-143 | Phytonadione | PHYTONADIONE |
| 52584-043 | PHYTONADIONE | phytonadione |
| 52584-046 | PHYTONADIONE | phytonadione |
| 0409-9157 | Vitamin K1 | PHYTONADIONE |
| 0409-9158 | Vitamin K1 | PHYTONADIONE |
| 50090-4521 | Vitamin K1 | PHYTONADIONE |
| 50090-4523 | Vitamin K1 | PHYTONADIONE |
Trademark Results [AquaMEPHYTON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
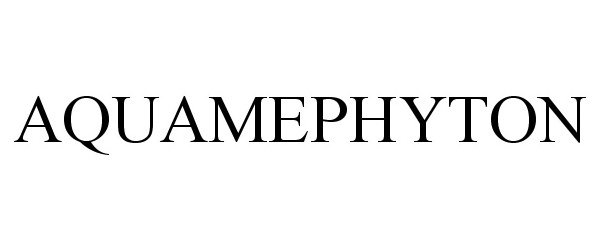 AQUAMEPHYTON 87924986 not registered Dead/Abandoned |
Teligent, Inc. 2018-05-17 |
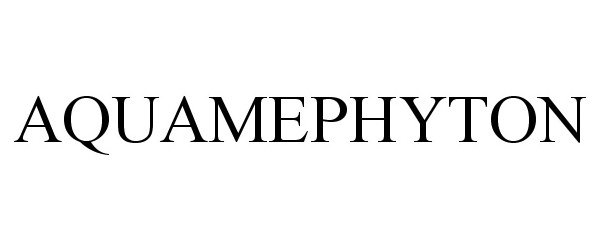 AQUAMEPHYTON 86494924 not registered Dead/Abandoned |
TELIGENT, INC. 2015-01-05 |
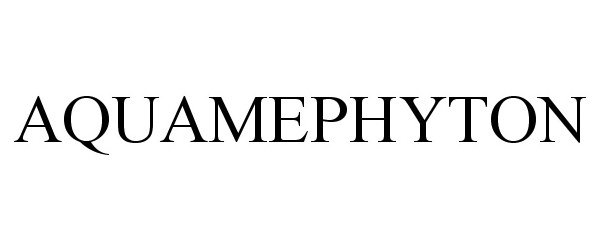 AQUAMEPHYTON 85051622 not registered Dead/Abandoned |
Aton Pharma, Inc 2010-06-01 |
 AQUAMEPHYTON 72071135 0689024 Dead/Expired |
MERCK & CO., INC. 1959-04-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.